How Juul's Teen Success Attracted Vaping Regulation
Juul Is So Hot It’s Set the Vaping Debate on Fire
(Bloomberg) -- The developers of the Juul e-cigarette wanted to make the experience of getting a stimulating hit of nicotine dramatically better than sucking on a stinky, smoking stick of burning tobacco. Their success made Juul the top-selling e-cigarette in the U.S. in two years. The company that makes the device says it was created to help adult smokers quit. But it achieved success in part by attracting a huge following among kids younger than 18, who aren’t legally allowed to purchase such products. Concerns about the hazards of vaping for the young provoked the U.S. Food and Drug Administration to announce plans Nov. 15 to sharply limit sales of tobacco products it says are designed to appeal to them.
1. What’s a Juul?
It’s a so-called vaping device containing a battery that heats nicotine liquid. The user inhales nicotine, an addictive alkaloid present in tobacco, and exhales aerosol. There’s no burning tobacco and thus no smoke or tar. The Juul has a sleek design. It’s made of brushed aluminum and resembles a USB flash drive. Because it’s small, the underage vaper can palm it, discreetly take a hit when a teacher or parent isn’t looking, and breathe the aerosol into a sleeve or collar. And, like many other vaping devices, its refills come in tasty flavors such as mango and creme.
2. How popular is the Juul?
Sales of the devices in the U.S. rose more than 600 percent in a year to 16.2 million in 2017, according to the Centers for Disease Control and Prevention. By the end of 2017, Juul accounted for almost 1 in 3 e-cigarettes sales, CDC found. Juul’s dollar share of such sales soared to 53 percent from 16 percent at the end of 2017, according to data from market researcher IRI. Reynolds American Inc.’s Vuse is next biggest with just 10 percent.
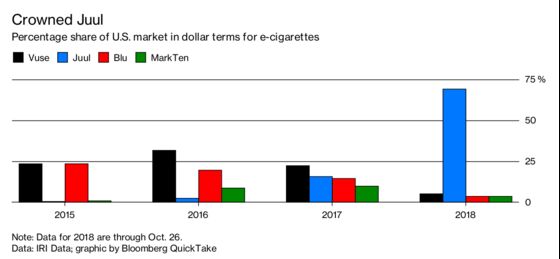
3. How common is teen vaping?
Scott Gottlieb, commissioner of the Food and Drug Administration, has described it as an epidemic. According to a U.S. government survey, vaping among high-schoolers rose 78 percent from 2017 to 2018. That meant that about 21 percent of those students were indulging. Among middle-schoolers, the number who reported vaping rose 48 percent, to almost 5 percent of the group. These increases meant that overall use of tobacco products among young people increased 38 percent, reversing declines in recent years.
4. What are the concerns?
Gottlieb put it this way: “The technology that might help adults end one addiction cannot pull a generation of kids into a new one.” While the evidence so far suggests that vaping is a safer choice than lighting up, there isn’t enough long-term data to make a definitive conclusion. It’s plausible, although not proven, that e-cigarette aerosols can damage tissue and cause disease, including cancer. The effects on humans of nicotine are not well-studied, although adolescents appear to be particularly vulnerable to it, with some evidence suggesting it can harm brain development. A report by the U.S. National Academies of Sciences said there was substantial evidence that young vapers are more likely than nonvapers to try regular cigarettes.
5. What has the FDA done?
It announced plans to restrict sales of most types of flavored e-cigarettes to specialized vaping stores and online retailers who verify a purchaser’s age. Tobacco, menthol and mint flavoring would be exempt because of research showing they’re more popular with adults than kids. FDA staff will next draw up the policies, which will be subject to public comment and could face court challenges from the industry. The planned curbs marked a change for the FDA, which a year earlier had pushed back by four years until 2022 regulation of vaping devices beyond a current ban on sales to minors and a requirement for nicotine-addiction warnings. Gottlieb said then that he wanted to ensure an industry with the potential to reduce smoking rates wasn’t stymied by regulation. The explosion in teen vaping altered Gottlieb’s plans. In what the FDA called the largest coordinated enforcement effort in its history, the agency over the summer issued more than 1,300 warning letters and fines to retailers that illegally sold e-cigarettes to minors. In September, FDA inspectors visited Juul Labs Inc.’s San Francisco headquarters and took away more than 1,000 pages of documents on sales and marketing.
6. What else might the FDA do?
Gottlieb said the option to take all e-cigarettes off the market is still on the table if the trends surrounding youth use don’t change.
7. What has Juul Labs done?
Just days before the FDA announcement, the company announced measures that were largely in line with the agency’s planned restrictions. It said that it temporarily had stopped selling to stores its nicotine pods in flavors mango, fruit, creme and cucumber and was providing only tobacco, menthol and mint flavors. The measure was expected to cut Juul’s in-store retail sales by 45 percent, according to a person familiar with the company’s projections. Juul said it would continue to sell the fruity pods through its website, but the company said it was adding age-verification systems to ensure customers are at least 18. It said it would resume selling flavored products to retail outlets that use age-verification technology.
8. How have other e-cigarette makers responded?
Altria Group Inc. earlier announced that it was temporarily pulling its e-cigarettes MarkTen Elite and Apex by MarkTen from the market until the FDA gives the green light. It said it will also stop selling Nu Mark cigarette alternatives in flavors other than tobacco, menthol and mint until granted the agency’s go-ahead. Altria, Reynolds and Juul Labs have all said they would support legislation to raise the legal age for tobacco buyers to 21.
The Reference Shelf
- The review of vaping studies by the U.S. National Academies of Sciences, Engineering and Medicine and another commissioned by Public Health England.
- The FDA’s Center for Tobacco Products’ website gives its position on e-cigarettes.
- The Public Health Law Center’s interactive map shows e-cigarette regulation in each U.S. state.
- A Bloomberg editorial on the dangers of vaping.
To contact the reporters on this story: Anna Edney in Washington at aedney@bloomberg.net;Sophie Alexander in New York at salexander82@bloomberg.net;Olivia Zaleski in San Francisco at ozaleski@bloomberg.net
To contact the editors responsible for this story: Drew Armstrong at darmstrong17@bloomberg.net, Lisa Beyer, Timothy Annett
©2018 Bloomberg L.P.