Crispr, the Tool Giving DNA Editing Promise and Peril
Gene Editing
(Bloomberg) -- Mankind has been manipulating genetics since early civilizations realized that certain traits of crops, animals and humans themselves were hereditary. The modern-day mapping of all human genes raised the prospects of learning precisely which genes control which traits and then directly altering their DNA codes. After years of hit-and-miss efforts, a gene-editing system called Crispr that's cheap, effective and easy to use promises to change our relationship with genetics — for better, worse or both. Its champions foresee using Crispr to control pests, increase food production and eliminate human diseases. They simultaneously worry that its use could unleash dangerous mutants, designer babies and new weapons of mass destruction. In the meantime, Crispr has given birth to a new biotechnology industry that is beginning to show promise in treating some intractable illnesses.
The Situation
Because of Crispr's ability to cut and paste individual genes, companies have been working to use the technology to rectify DNA flaws that lead to inherited disease. Those efforts gained a strong push in late June when drugmakers Intellia Therapeutics Inc. and Regeneron Pharmaceuticals Inc. reported results from the first clinical trial using Crispr technology to treat disease inside the human body. In the early-stage trial of just six people, researchers used Crispr to edit out a flawed gene that results in production of an abnormal liver protein that accumulates throughout the body, sometimes causing severe and even lethal symptoms. While the study doesn’t prove the approach works, the patients who were treated saw significant reductions in levels of the harmful protein. In the past, gene editing had been used to alter human cells that were removed from the body and then replaced; the companies’ trial showed that that step isn’t always needed. Additional companies, such as Editas Medicine, are also working on similar projects. Other researchers have used Crispr inside the body, but not to treat disease. Chinese scientist He Jiankui announced in 2018 that he’d used the technology to alter the genes of a pair of twins while they were embryos with the aim of making them resistant to HIV. Using Crispr to make changes to embryos and germline cells — sperm, eggs and zygotes — is especially contentious because the modifications are passed to progeny. His announcement resulted in an ethical outcry and further restrictions on manipulating the DNA of healthy embryos.
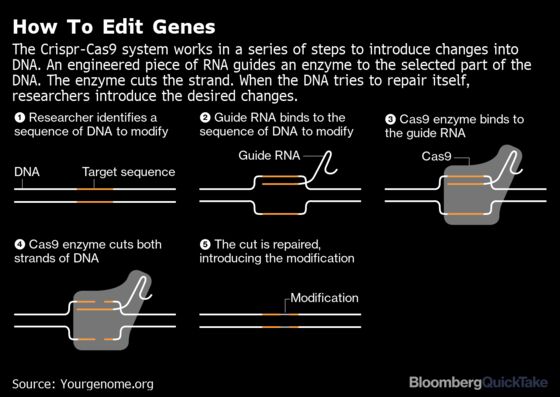
The Background
Crispr technology is based on a rudimentary immune system that Japanese scientists first noticed in bacteria three decades ago and named Clustered Regularly Interspaced Short Palindromic Repeats. These sequences of genetic code destroy pathogens by cutting the DNA of the invader using enzymes called CAS nucleases, Cas9 being the most widely studied. Understanding of how the system can chop through and then replace segments of DNA grew slowly until 2012, when researchers at the University of California, Berkeley published a paper on making molecular “guides” that allow Crispr to skim along DNA, targeting exactly the right spot to make a slice. Soon afterward, scientists at the Broad Institute in Cambridge, Massachusetts said they’d adapted Crispr for use in human cells. In 2020, pioneering scientists Jennifer Doudna and Emmanuelle Charpentier were awarded the Nobel Prize in Chemistry for their development of the gene-editing technology. A researcher with basic skills and just a few hundred dollars’ worth of equipment can employ Crispr, creating enormous space for innovation, and abuse. The gene-editing system isn’t perfect. It makes unintended cuts in DNA, with effects unknown. Scientists are working on minimizing these slip-ups. A newer method of gene revision, called prime editing, is thought to produce fewer unwanted alterations.
A Bloomberg video explores the transformative power of Crispr
The Argument
While Crispr offers enormous potential to improve human welfare, some of the risks are also immense. By improving so-called gene drives, experimental systems that increase the chance a certain gene is inherited, Crispr might one day, for instance, ensure that mosquitos can no longer host the Zika virus. Yet theoretically, the modifications could also allow the bugs to spread a more harmful pathogen. Germline editing raises similar issues. Potentially, a genetic disease could be eliminated from a family forever. But if something goes wrong, the consequences are potentially eternal, too, affecting future generations who would not have given their consent to the intervention. Some scientists worry that germline editing would invite enhancements of babies for non-medical reasons and could even lead to the division of humans into subspecies. Other commentators have argued that people bred to be supersmart could produce positive effects for society by generating innovations that would be used by everyone. Meanwhile, defense specialists fret over the possible military applications of Crispr. In its 2019 assessment of worldwide threats, U.S. intelligence agencies warned of adversaries potentially using gene editing to “develop novel biological warfare agents, threaten food security, and enhance or degrade human performance.”
The Reference Shelf
©2021 Bloomberg L.P.