J&J Shot to Get EU Nod in Early March, Easing Supply Squeeze
J&J Shot Set to Get EU Nod in Early March, Easing Supply Squeeze
(Bloomberg) -- The European Union’s medicines regulator is set to recommend Johnson & Johnson’s coronavirus vaccine early next month, bolstering supplies even as governments struggle to get shots into people’s arms.
The European Medicines Agency’s nod is expected on March 11, an EU official said on condition of anonymity. It would clear the way for market authorization of a fourth Covid-19 vaccine, alongside those from Moderna Inc., AstraZeneca Plc and a partnership of Pfizer Inc. and BioNTech SE.
An EMA spokesperson said the agency is working toward issuing an opinion by mid-March but it couldn’t confirm a date. The EU official also said EMA talks are ongoing with Russian authorities on the Sputnik V vaccine and may receive data soon to begin a rolling review process.
The European Commission has said deliveries of the J&J shot are expected to begin in early April, adding to a surge in supplies over the second and third quarter. President Ursula von der Leyen showed a slide to EU leaders during a video summit Thursday projecting a dramatic increase in vaccine deliveries over the coming months.
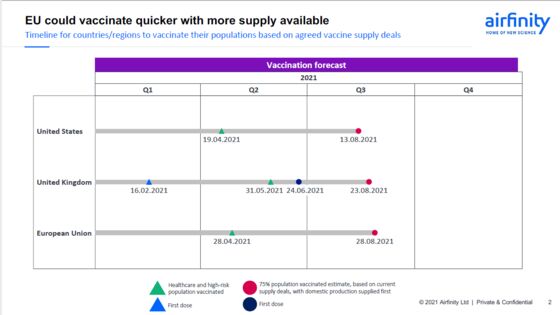
While some leaders, including Italian Prime Minister Mario Draghi, have said they aren’t convinced about the reliability of such forecasts, independent analysis from London-based research firm Airfinity Ltd. shows that EU supplies should suffice to vaccinate 75% of the region’s adult population by the end of August.
The EU’s advance purchase deal with J&J allows member to purchase vaccines for 200 million people, with an option for another 200 million. The EMA is also carrying out a rolling review of the CureVac NV and Novavax Inc. shots. The Commission has struck deals for the initial purchase of 225 million CureVac shots and concluded exploratory talks with Novavax to purchase 100 million doses.
The increase in deliveries puts the onus on EU governments to speed up inoculations. Governments have attributed the underwhelming rollout to delays in the procurement of shots, which has been managed by the commission, and the EMA’s slow approval process.
In the U.S., a committee of advisers to the Food and Drug Administration is meeting to review J&J’s shot on Friday.
Data shown by Von der Leyen to leaders illustrate that more than 20 million shots delivered to the EU’s 27 member states have yet to be administered, raising doubts about the national health authorities’ ability to handle the looming supply surge and mobilize the resources needed to ramp up vaccinations.
Questions around the efficacy of the shot from AstraZeneca and its partner, the University of Oxford, and restrictions on its use among the elderly in many EU countries have fueled skepticism. Vast stocks of the vaccine have been left unused in France and Germany.
Trailing the U.S., U.K.
The EU lags behind the U.S. and the U.K. in its inoculation campaign, having delivered just 6.8 doses per 100 people, according to Bloomberg’s global tracker. But performance varies between member states, with Denmark delivering about four times as many doses as Bulgaria per 100 people, even though each government has access to the same number of shots relative to its population.
Although about 33% of all vaccines delivered to the EU have gone unused, Denmark is using every drop available. It’s also aiming to immunize all adults by the end of June, faster than the EU-wide target of Sept. 21.
If other countries are leaving unused vials, Denmark is happy to purchase more, Prime Minister Mette Frederiksen said.
“We will very much like to bring them to Denmark,” Frederiksen told reporters in Copenhagen on Friday. “And we will also pay a good price for the vaccines.”
©2021 Bloomberg L.P.