Five Things to Watch in European Health-Care Stocks for 2020
Five Things to Watch in European Health-Care Stocks for 2020
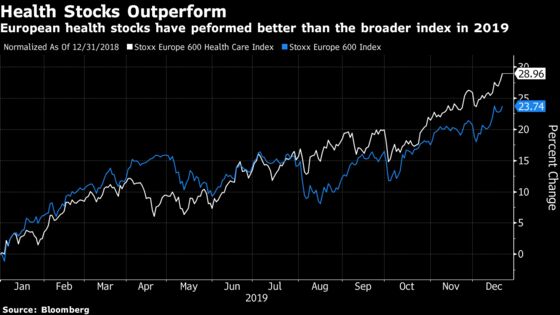
(Bloomberg) -- European health-care stocks are on track for their best year since 1997, and analysts see plenty of reasons for the momentum to continue in 2020 despite the inevitable “noise” surrounding drug costs and pricing in a U.S. presidential election year.
Areas to watch include diversification of product pipelines, expansion in China and innovation with new technologies, particularly in medical equipment. European drugmakers also are likely to benefit from investors’ shift into defensive sectors as the economic backdrop remains weak, said Bloomberg Intelligence analyst Sam Fazeli, who sees double-digit earnings growth at companies including AstraZeneca Plc and Novo Nordisk A/S.
Pricing Peril?
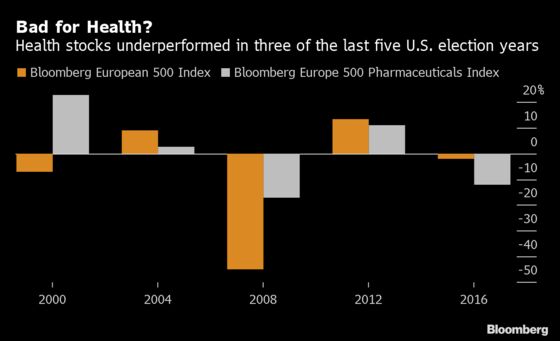
Health care has long been a key point of contention in U.S. elections and 2020’s presidential contest looks set to be no different. Debate over pricing and reimbursements in the Medicare insurance program for the elderly could pressure stocks, particularly those of larger drug companies. But the rhetoric may be worse than the reality, at least in the short term. While “there’s going to be a lot of noise” in any presidential debates, in reality it’s hard to get reforms passed, Shore Capital’s Adam Barker said in an interview.
A proposal to change Medicare’s prescription-drug benefit, known as Part D, is among the reforms that’s most likely to become law in 2020, which could hurt drugmakers’ ability to raise prices.
AstraZeneca and Novartis AG are the most exposed to one bipartisan bill, the Prescription Drug Pricing Reduction Act, due to the level of high-priced oncology drugs in their portfolios, said Peter Welford of Jefferies. There also will be renewed discussion of indexing drug prices to international levels, though practical issues in creating such an index make the proposal “a complete non-starter,” he said.
Chasing China
The heat may be rising in the U.S., but it’s becoming more temperate in China, which has opened its market to Western drugs. An increased respect for intellectual property and faster timetables and review cycles are among the developments adding to the region’s attractiveness, according to Jefferies.
At the end of November, some 70 new therapies were added for coverage by China’s state-run medical insurance fund after months-long negotiations over prices, including AstraZeneca’s anemia medicine roxadustat and Roche Holding AG’s lung cancer treatment alecensa. Shore’s Barker particularly noted AstraZeneca’s strength in China, and Jefferies’ Welford views it as “the best positioned of EU pharma companies” in the country given future growth opportunities.
Research Developments
While an uncertain macro environment may be supportive of the stocks, a highly productive period in research and development is expected to be a fundamental driver of the sector. Barclays analyst Emily Field said in an interview she expects more positive returns on R&D spending in the longer term, with a number of significant data readouts expected in 2020.
Deutsche Bank anticipates 14 major new drugs reaching the market in 2020, with potential peak sales of about $13 billion. Launches to watch include Novartis’s Beovu for macular degeneration, GlaxoSmithKline’s multiple myeloma drug belantamab mafodotin and the full commercial introduction of Novo Nordisk’s Rybelsus for diabetes, analysts said. Regulatory reviews of AstraZeneca’s breast-cancer drug DS-8201 and roxadustat for anemia will be key to determining sales potential, according to Bloomberg Intelligence.
There will be challenges too, however, as drugs become more genetically specialized, meaning smaller patient populations and lower peak sales, according to Barker. As payers continue to be focused on value-based outcomes, there’s an increasing demand for “evidence that the drugs actually work,” he said.
Innovation Amid Regulation
While politics is a potential drag on pharma stocks, the regulatory outlook is clearer in health equipment. “We believe medical devices are in a very different place than pharma today, as the cost of medical devices are not generally in the political crosshairs,” Bernstein analyst Lisa Bedell Clive wrote in a recent note.
R&D “remains paramount” with innovation -- driven by expansion in artificial intelligence, connectivity, and robotics -- becoming increasingly relevant, she said. Clive sees Royal Philips NV and Siemens Healthineers AG as leaders in artificial intelligence and big data, since they can capitalize on vast imaging archives, while Smith & Nephew Plc has been a pioneer in using robotics.
New rules -- European Union regulations that come into force in May and U.S. Food and Drug Administration plans to modernize monitoring processes -- also will require investment in research and technology.
Bolt-on Bolstering
As always in health care, mergers and acquisitions could be another source of growth, particularly among mid-sized companies. Hikma Pharmaceuticals Plc and Vifor Pharma AG have both indicated they may be more active on the M&A front in the year ahead, Barclays’ Field said.
Deutsche Bank also says it expects “bolt-on deals to bolster internal innovation” remaining the focus for M&A with Roche, Novartis and Sanofi the most likely dealmakers.
Deal-making in the year ahead “probably won’t be to the same extent as this year,” Field said, with the broad focus on deal execution after a busy year. “But who knows?”
To contact the reporters on this story: Morwenna Coniam in New York at mconiam@bloomberg.net;Erin Roman in London at eroman16@bloomberg.net
To contact the editors responsible for this story: Beth Mellor at bmellor@bloomberg.net, Phil Serafino, Tom Lavell
©2019 Bloomberg L.P.