Trump Is Overhyping Unproven Coronavirus Drugs

(Bloomberg Opinion) -- President Donald Trump has cleared a low bar on coronavirus communication in the past week by finally acknowledging the seriousness of the outbreak, and endorsing needed social-distancing measures. A confusing press conference Thursday in which the he overhyped the promise and availability of new coronavirus treatments was a big step backward.
"So you have remdesivir, chloroquine and hydroxychloroquine," Trump said. "Those are out now, essentially approved for prescribed use. I think it could be a game changer, and maybe not. Very powerful, they're very powerful."
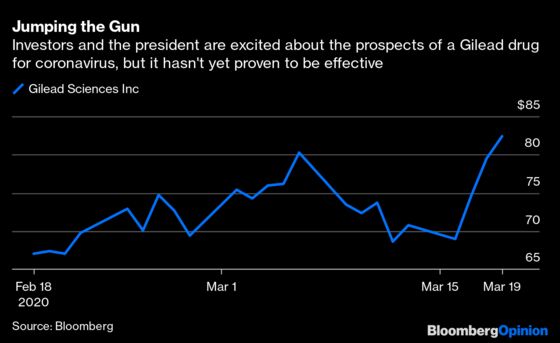
"Maybe not" might be the most accurate part of that sentence. Chloroquine, an older previously approved drug mostly used for malaria, hasn’t been approved for use in Covid-19 patients by the Food and Drug Administration. The same goes for Gilead Science Inc.'s repurposed ebola treatment Remdesivir. Neither are widely available to be prescribed to treat Covid-19, and both require further testing before that will happen. A treatment for the virus will be crucial to stopping its spread, but we aren’t nearly as close as the president would have you believe.
These drugs are currently usable only under limited circumstances, via clinical trials, if a physician applies to the FDA for "compassionate use" for individual cases, and if physicians write a so-called off-label prescription in the case of chloroquine. That's as it should be. Speed is essential in an outbreak, but shortcuts are dangerous.
These drugs are being discussed for a reason. They've been around for a while, so their safety profile is better understood — or as Trump put it Thursday, "we know if things don't go as planned, it's not going to kill anybody. When you go with a brand new drug, you don't know that's going to happen." That's actually overstating things a bit; negative effects can emerge when drugs are used more broadly or in different populations. And while it’s true that the history of these drugs could justify a quicker approval, that won’t — and shouldn’t — happen until they prove their worth in large and rigorous clinical trials.
Remdesivir is being tested in China right now, but those trials are still "blinded." That means even the researchers involved haven't seen data indicating the drug works, let alone shared it. The proof that it works comes from essentially anecdotal evidence from a few patients. Nothing more is due for at least a few weeks. There have been positive news stories about the use of chloroquine in France, but they focus in large part on a tiny and poorly controlled study.
For the president, the limited evidence available is apparently enough to say that chloroquine "could totally depress any time that we talk about" referring to the length of the outbreak and that it "is going to be available by prescription."
Luckily, there were cooler heads in the room. The guy who knows what he's talking about, FDA Commissioner Stephen Hahn, clarified at the press conference that his agency is just taking a "closer look" at chloroquine and that a trial will be run. In contrast with Trump's bombastic and misleading statements, his message was that "it's important not to provide false hope, but to provide hope. As a doctor, that's how I come to this."
That’s the right idea. Everyone should hope that these drugs work, and they should be sped to market if they do. But no one should be relying on them yet.
This column does not necessarily reflect the opinion of Bloomberg LP and its owners.
Max Nisen is a Bloomberg Opinion columnist covering biotech, pharma and health care. He previously wrote about management and corporate strategy for Quartz and Business Insider.
©2020 Bloomberg L.P.