The Novel Approach to Testing a Covid-19 Vaccine From France
The Novel Approach to Testing a Covid-19 Vaccine From France
(Bloomberg) -- Valneva SE is looking at running a head-to-head trial with an approved Covid-19 vaccine for advanced tests of its own shot in the U.K., where the rapid rollout of immunizations could make it hard to conduct a conventional study.
The French pharmaceutical company is in discussions with regulators about whether volunteers in the control arm of the trial, planned to start in April, could be given a coronavirus shot that has already been authorized, Chief Executive Officer Thomas Lingelbach said in an interview.
If the health authorities agree, the company would be the first to run such a trial prior to approval of its Covid-19 vaccine. As shots become more widely available, Valneva considers it wouldn’t be ethically justifiable to run a trial comparing its injection to a placebo, the standard approach for medical trials, because it would require participants in the control group to forgo possible protection.
In addition, as more people get vaccinated, that could lead to fewer new Covid infections, which could slow down results. Trials conclude once a certain proportion of participants are diagnosed with the disease, allowing researchers to gauge a shot’s effectiveness and safety.
“We have right now five Covid vaccines that have shown efficacy” that Valneva could pick from as a comparator, Lingelbach said.
4,000 Participants
The company is planning to enroll about 4,000 people in its late-stage trials in the U.K. and aims for approval in the last quarter of 2021. Given the government’s target to vaccinate everyone over 70 by mid-February, the company may have to do a smaller, separate trial for older adults in another geography later if it can’t recruit enough participants in Britain, according to Lingelbach.
Other drugmakers have been joining forces to speed up the production of vaccines as governments push for more capacity to ease the crisis. Valneva is unusual among smaller vaccine makers because it doesn’t have a big pharma partner.
“Will we seek a partnership? It depends a little bit on how we’re going to look at the Covid business in the long run,” Lingelbach said in a separate interview with Bloomberg Television on Friday. “Right now, we’re focusing on the pandemic and the pandemic response. Whether Covid vaccinations will be needed beyond 2022 or not will also determine our commercial strategy.”
Valneva shares soared 17% in Paris on Thursday as the European Union confirmed plans to finalize its preliminary agreement to buy doses from the company. The bloc has struggled to scale up its vaccination efforts alongside multiple delays from suppliers. On Friday morning the stock rose an additional 4%.
The U.K. government invested in the company’s Scottish manufacturing site last year to help scale up supplies and this week exercised its option to buy 40 million more doses of the shot. The site is currently operating at roughly 20% of its targeted capacity, and is expanding more with the support from the U.K. government, Lingelbach said.
Britain has bought 100 million doses of the vaccine in total, with an option for another 90 million from 2023. The EU, which has agreements with vaccine makers including AstraZeneca Plc, Johnson & Johnson and Pfizer Inc., will pay slightly more than the roughly 7 euros ($8.4) a dose the U.K. is paying, given that that country is investing in the development, Lingelbach said.
Valneva started early-stage trials in December and is one of the few companies in the West developing an inactivated shot against the SARS-CoV-2 virus. The approach has been used for decades and involves using a sample of the virus that has been killed to stimulate an immune response without causing the disease.
Scotland, Sweden, Austria
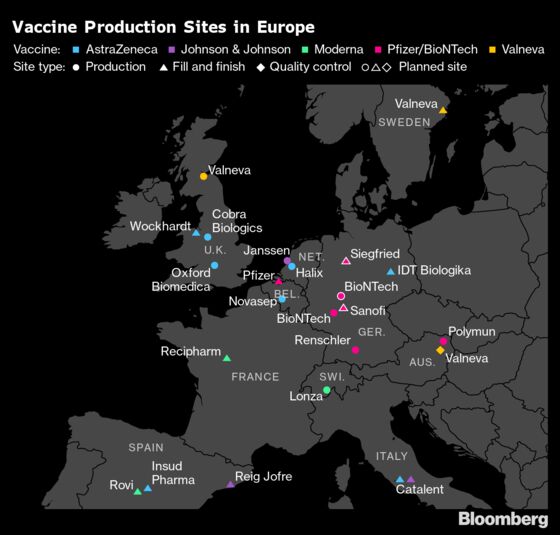
The drug substance will be produced at the company’s Scottish site before being sent to Sweden to be bottled and packaged and then to Austria for quality-control checks. Lingelbach said there’s little the company could do to change manufacturing routes if the EU clamps down further with its vaccine export controls.
With other inactivated vaccines in China reporting positive data, Lingelbach is optimistic the vaccine will be successful.
We knew “we would never be the first-line product but we had always hoped that we could play a complementary role in the portfolio of different vaccines,” he said.
©2021 Bloomberg L.P.