U.K. Backs Pfizer Covid Shots for Vulnerable Young Children
U.K. Allows Pfizer Covid Shot for At-Risk Children Aged 5 to 11
(Bloomberg) -- The U.K. vaccines panel cleared the Pfizer Inc. Covid-19 shot for use in vulnerable young children in a bid to widen vaccination coverage against the omicron variant.
The Joint Committee on Vaccination and Immunisation changed its advice to allow at-risk children aged 5 to 11 years old to become eligible for two doses of the Pfizer-BioNTech vaccine. Each inoculation will be one-third of the dose used for those aged 12 and above.
Only children in a clinical risk group or who are a household contact of an immunosuppressed person are eligible for vaccination at this stage. The JCVI said it would issue advice on shots for less vulnerable children “in due course.”
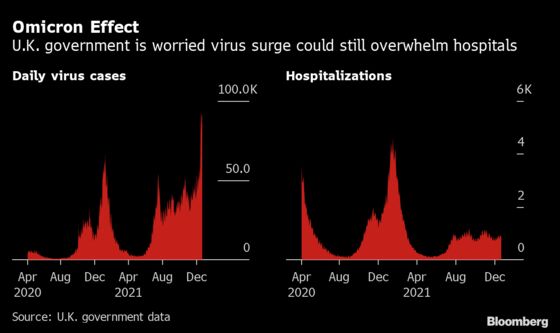
The move comes at a key moment in the pandemic as the U.K. grapples with soaring infection rates from the new omicron variant. While children haven’t suffered from Covid-19 as severely as adults, the number of cases in the group has been growing due to their unvaccinated status. That’s raised fears of transmission to grandparents and vulnerable adults over the Christmas period.
U.K. officials said they were prioritizing vulnerable children, initially giving the Pfizer vaccine to the limited group of 5 to 11 year-olds, because the benefits of the vaccine are greater for that cohort.
“The majority of children aged 5 to 11 are at very low risk of serious illness due to Covid-19. However, some 5 to 11 year olds have underlying health conditions that put them at higher risk, and we advise these children to be vaccinated in the first instance,” JCVI Chair Wei Shen Lim said.
Advice for older children was also updated on Wednesday. All 16- and 17-year-olds can now be offered a booster dose, as can children aged 12 to 15 who are clinically vulnerable or immunosuppressed. Others aged 12 to 15 can receive two doses.
The panel is waiting to see more evidence on the efficacy of the vaccine against omicron, officials said. They will also monitor further safety data from countries that already administer the jab to children before making a recommendation on whether to expand its roll-out in Britain.
The JCVI’s decision came as the U.K. medicines regulator concluded that the Pfizer-BioNTech vaccine is safe and effective for children aged 5 to 11.
“Our detailed review of all side-effect reports to date has found that the overwhelming majority relate to mild symptoms, such as a sore arm or a flu-like illness,” said June Raine, chief executive of the Medicines and Healthcare products Regulatory Agency.
The U.K. and the JCVI have come under criticism for moving too slowly on Covid-19 vaccines for children, a move that contrasts with its high-speed approach to vaccinating adults. While the U.S. and most of Europe started offering shots to adolescents in the spring and summer the U.K. waited until September, and then only offered shots on a limited basis.
©2021 Bloomberg L.P.