Tiny U.K. Company’s Stock Soars 552% as Drug Cuts Covid Risk
The company said its experimental drug cut the risk of developing the worst symptoms of Covid-19.

(Bloomberg) --
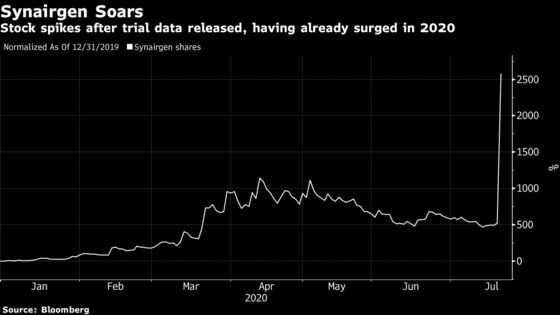
Shares of Synairgen Plc, a tiny pharmaceutical firm born out of a U.K. university, soared as much as 552% after the company said its experimental drug cut the risk of developing the worst symptoms of Covid-19.
In a clinical trial involving 220 subjects, the probability of a patient requiring ventilation or dying dropped 79% for those who got Synairgen’s SNG001 versus those who received a placebo, the Southampton, England-based company said in a statement. Those receiving SNG001 also were more than twice as likely to fully recover from the illness, it added.
The next steps are to complete analysis of the data, including safety results, Chief Executive Officer Richard Marsden said on a call with journalists. From there, the company will enter into discussions with regulatory agencies to move toward approval, he said.
“We are expecting greater international interest because of the data that’s been produced today,” Marsden said.
The inhaled product is a formulation of a naturally occurring protein, known as interferon beta, that Synairgen has previously said may be deficient in those with greater susceptibility to the new coronavirus, such as the elderly or those with heart and lung complications or diabetes.
Synairgen rose 430% to 193.50 pence at 3:13 p.m. London time. The stock’s latest surge takes its year-to-date gain to about 3,194%, giving Synairgen, which trades on London’s AIM venue for growth companies, a market capitalization of about 289.2 million pounds ($365 million).
“Valuing the Covid-19 opportunity is nigh on impossible,” FinnCap analyst Mark Brewer said in a note to clients, adding that upcoming discussions with regulators should clarify SNG001’s route to market. FinnCap is also corporate broker to the company.
Three patients who received placebos in the study died, while there were no deaths among subjects treated with SNG001, according to the company.
Synairgen, founded by three professors from the University of Southampton, previously partnered with AstraZeneca Plc in 2014 to study SNG001 in severe asthma, but the trial was halted two years later after an interim analysis showed a very low number of reported severe exacerbations, making it difficult to draw conclusions.
©2020 Bloomberg L.P.