Some Patients’ Best Hope for a Cure Is to Develop It Themselves
Some Patients’ Best Hope for a Cure Is to Develop It Themselves
(Bloomberg) -- It was a life-or-death situation for young Matthew Evangelista. His mother, Helen, had nowhere to turn.
Doctors couldn’t help. Matthew was born with a disease that affects only 200 people in the world, and there was no treatment. With such a small number of patients, pharmaceutical companies saw no profit in developing a drug therapy to fight it.
So it was up to Mom to save his life.
That’s how it goes for many people with ultra-rare diseases, defined as affecting 20 or fewer people per million. At that rate, the estimated 6,500 Americans who suffer from such conditions can’t bank on a celebrity spokesperson or a national TV telethon to raise awareness and money for research. Many have to rely on their families to advocate for them.
Parents can spend countless hours becoming experts on their loved ones’ afflictions and push for possible cures in the hope that someone, somehow, will produce a life-saving therapy. Even for parents with Wall Street resources and a web of connections, the process can be daunting.
Amber Freed is using the skills she honed as an equities analyst at Janus Henderson Group Plc to save her son, Max. At the time of his diagnosis, he was one of only about three dozen people in the U.S. known to have a genetic condition, SLC6A1, that could be fatal if left untreated. She's decided her only hope is to bypass the pharmaceutical industry and fund the research on a new gene therapy directly. But she needs cash.
“This is what’s so frustrating,” Freed said. “It’s truly a matter of money.”
Ilan Ganot was an investment banker at JPMorgan Chase & Co. in London when he learned that his toddler suffered from Duchenne muscular dystrophy, a deadly muscle-wasting disease. With support from bank colleagues, including Chief Executive Officer Jamie Dimon, Ganot started his own biotechnology company, Solid Biosciences Inc., which is now a public company. He said he was motivated in part because he knew he had resources and connections other parents didn’t.
“It's not for everyone just to start a biotech,” Ganot said in an interview. “I was fortunate that I could take some personal risk and even more fortunate to be able to walk up to Jamie Dimon and have him say, ‘Yeah, let's do something about this disease.’”
Some parents without Ganot’s resources band together. The National Organization for Rare Disorders represents nearly 300 separate patient foundations. While many advocacy groups are bankrolled by the drug industry and in some cases are even public relations fronts, groups for ultra-rare diseases are often too small to attract the interest of Big Pharma and have to fund basic research themselves.
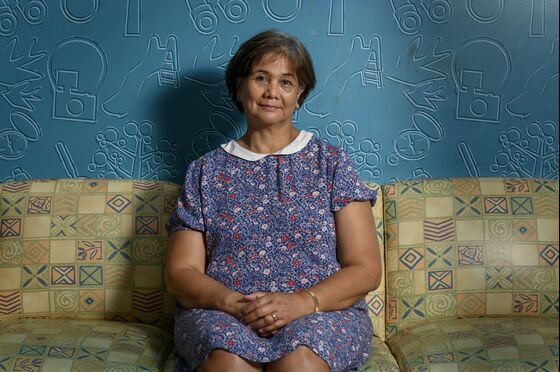
For Helen Evangelista, an immigrant from the Philippines who makes $20 an hour as a hospital clerk in Brooklyn, New York, it took years to find a way to combat Matthew’s metabolic disorder, called MPS 7 or Sly syndrome. He was diagnosed as a baby.
Matthew will turn 18 in October.
“Fortunately for Helen and Matthew, it worked out,” said Emil Kakkis, chief executive officer of Ultragenyx Pharmaceutical Inc., the company that makes Matthew’s treatment. “It doesn’t work out for a lot of people.”
Kakkis said he fields at least one or two calls a week from parents whose children have recently been diagnosed with a mystery condition. His company is one of the few that researches disorders that may only have hundreds of patients. There are more ailments than the company could ever possibly research, he said, and often the best help he can give parents is teaching them how to get treatments developed themselves.
The economics of ultra-rare diseases are daunting. Mepsevii, the drug Matthew Evangelista takes, costs $375,000 a year. Helen Evangelista calls Ultragenyx “the only hope that I had.”
But even in the case of Mepsevii, the numbers show why so few companies are interested in ultra-rare diseases. The drug brought in about $8 million last year. That’s a pittance in biotechnology.
“Each product still has to be profitable within three years,” Kakkis said. Ultragenyx, founded in 2010, is not yet profitable and is estimated to lose more than $350 million this year.
Mepsevii was first created more than 20 years ago by William Sly. For most of that time, it sat on a shelf in Sly’s St. Louis University lab. Locating it and matching it with Matthew Evangelista was Helen Evangelista’s quest.
When Matthew was a toddler, Helen Evangelista found Kakkis, a physician and biotech executive who developed a drug for a related syndrome, MPS 1. Kakkis offered to attend a walkathon the Evanglistas were holding in Queens. That’s where he first met Matthew, already confined to a wheelchair.
“I told them we would try to do something but I didn’t really know what would happen,” Kakkis said.
With so little potential profit, Kakkis said he couldn’t drum up interest in Sly’s compound at BioMarin Pharmaceutical Inc., the biotechnology company where he worked at the time.

Helen Evangelista persisted. She wouldn’t let Kakkis forget Matthew.
A breakthrough came in 2010. Kakkis left BioMarin to form his own company, Ultragenyx, to develop drugs for ultra-rare conditions. With thousands of disorders worthy of research, it was Helen Evangelista’s tenacity that kept Matthew’s story on his mind.
Kakkis was able to work on the drug, and by 2013 the company was ready to begin testing in patients.
It didn’t come a moment too soon. At 12, Matthew’s mother and his doctors believed he was dying. The boy received treatment with Kakkis’s new drug through a compassionate-use program, which allows patients with no other options access to unapproved medicines.
Within days, Matthew’s symptoms improved. He was the first patient in the first clinical trial of Mepsevii. Five years later, while still unable to speak, walk or breathe on his own, he’s alive.
The tools, like gene therapy, to address diseases have evolved in the past 20 years, meaning the science is not necessarily the hurdle for some conditions, Kakkis said.
“The problem is the ultra-rares get ignored, though science we’ve developed could very well treat them,” he said. “The tragedy is if we develop the science to treat something and don’t do it, what’s wrong with us?”
To contact the editor responsible for this story: Drew Armstrong at darmstrong17@bloomberg.net, Bob Ivry
©2019 Bloomberg L.P.