Philip Morris's IQOS Seen Benefiting From FDA Flavor Crackdown
Philip Morris's IQOS Seen Benefiting From FDA Flavor Crackdown
(Bloomberg) -- The unintended consequence of the U.S.’s decision to combat teen vaping could be a bump for IQOS, made by traditional cigarette maker Philip Morris International Inc., one analyst says.
IQOS, a “heat-not-burn” product, is already available in Japan, Korea and some other markets, and an application to sell it in the U.S. is being considered by the Food and Drug Administration. PMI seeks to market it as a “reduced-risk” product for regular smokers. Altria Group Inc., which spun off Philip Morris in 2008, would also benefit from any IQOS success, since it would be the one marketing the device to American smokers.
If the FDA moves to reduce menthol in cigarettes over time, IQOS could benefit, said Wells Fargo analyst Bonnie Herzog. That’s because the FDA may opt to allow menthol flavored e-cigs for some time in order to convert more smokers to the reduced risk products, she said.
“In fact, we see IQOS playing a pivotal role in this process and as such should be approved promptly given its tobacco & menthol (not mint) flavor variants,” Herzog said in a Thursday research note. The device could accelerate the conversion of customers of menthol brands like Newport, taking incremental share, and is less attractive to youth, she said.
Her bottom line: It’s a good time to buy Altria shares because IQOS appears to be coming for the U.S. market.
Philip Morris Chief Financial Officer Martin King told Bloomberg News in an interview earlier this week that the product’s taste and “ritual” was designed specifically to help convert cigarette smokers. The two companies worked on the product together before the spin-off, he said.
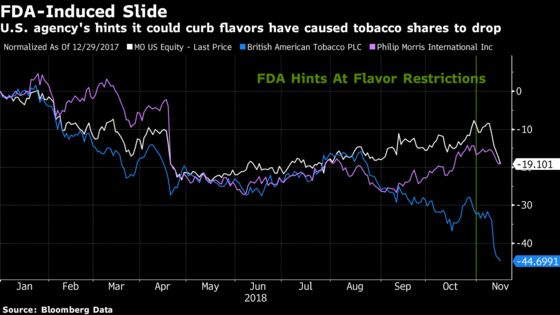
Shares of Philip Morris, which sells Marlboros in overseas markets, have fallen 19 percent this year. Stock of Altria, which sells the brand in the U.S., has declined by a similar amount.
Philip Morris Chief Executive Officer Andre Calantzopoulos said this week he’s “optimistic” the company will hear from the FDA on IQOS by end of the year.
To contact the reporter on this story: Tiffany Kary in New York at tkary@bloomberg.net
To contact the editors responsible for this story: Anne Riley Moffat at ariley17@bloomberg.net, Jonathan Roeder
©2018 Bloomberg L.P.