Pfizer Fails to Best Sarepta With Early Data on DMD Gene Therapy
Pfizer Fails to Best Sarepta With Early Data on DMD Gene Therapy
(Bloomberg) -- Pfizer Inc.’s experimental gene therapy for boys with Duchenne muscular dystrophy helped treat the underlying cause of the deadly disease in a small study, though it also triggered side effects and may further stoke questions in the hotly contested field currently led by Sarepta Therapeutics Inc.
One of three boys given the highest dose in the Pfizer trial was admitted to the pediatric intensive care unit after he had an immune system reaction that caused kidney damage, destroyed red blood cells and led to low platelet counts. While he fully recovered after undergoing dialysis and drug treatment, Pfizer has paused the study until an updated safety monitoring system is approved. Another patient was hospitalized for two days because of severe vomiting.
Sarepta jumped as much as 20% to the highest intraday level since October on Friday, after the data was presented. Pfizer was little changed.
All six boys enrolled in the early-stage trial, who are age 6 to 12, started producing a key protein after getting the gene therapy. That raised the possibility that the one-time treatment may alleviate the condition, which is marked by progressive muscle weakness and an early death. While boys with DMD typically don’t make any of the dystrophin protein needed for muscles to work properly, those given Pfizer’s compound produced 10% to 60% of normal levels, the New York-based company said.
Although it’s still early, those results fell short of a compound developed by Sarepta. The Cambridge, Massachusetts company won a standing ovation a year ago when it unveiled promising results from its initial study. Three patients who participated in the trial walked out to gasps and cheers at a company-sponsored presentation in New York.
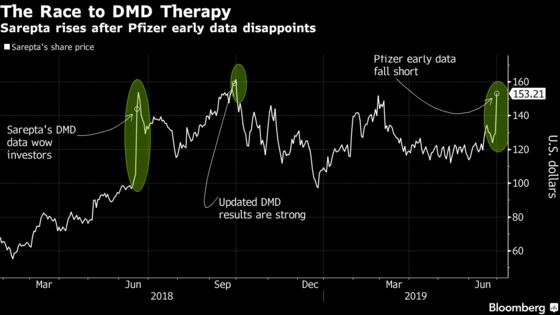
The Pfizer results were presented at the Parent Project Muscular Dystrophy Connect Conference in Orlando, Florida. The compound, known as PF-06939926, is the outside shell of a virus that is engineered to carry a truncated version of the gene that produces dystrophin. After it is given intravenously, the virus deposits the DMD gene inside the patient, where it begins to produce mini-dystrophin.
The significance of the findings may be difficult for doctors, families and investors to evaluate because Pfizer developed its own proprietary test to evaluate dystrophin production, rather than rely on a standard analysis called Western Blot that measures the level of dystrophin expression. Pfizer says that methodology is limited. Measured by Pfizer’s method, the average expression levels of mini-dystrophin produced two months after treatment ranged from 23.6% in three boys getting the lowest dose to 29.5% in those on the high dose.
The lack of common methodology makes comparisons more challenging. Still, Sarepta’s compound showed stronger results in its initial study: Three boys had an average expression of micro-dystrophin that was 38.2% of normal using the Western Blot test, with no significant safety concerns. Updated results that included a fourth boy in October showed an average micro-dystrophin expression of 74%.
Researchers found significant levels of dystrophin within the muscle fibers of boys given Pfizer’s gene therapy, at an average of 38% in the low dose group and 69% in the high dose group. The first two treated boys, who were 7 and 8 when they started the trial, posted gains on a standard test used to measure their ability to move, including walking, climbing and running. While boys typically plateau or start to lose their motor abilities at 7, the two in the trial posted a 4.5-point gain one year after treatment.
The side effects could raise concerns for other companies working on DMD. Solid Biosciences Inc. is studying an experimental gene therapy of its own, and safety concerns and minimal benefits have wiped away more than 85% of its market value in the last year. Trading of the stock seesawed Friday after Pfizer’s data was released, swinging up 18%, then dropping 11%. They were up 5% as of 10:51 a.m.
Pfizer said it plans to start a study from the third and final phase of drug development for the gene therapy in the first half of 2020. While the company halted enrollment after the patient on high dose went to pediatric intensive care, it still plans to eventually bring six additional boys in the ongoing trial.
To contact the reporters on this story: Michelle Fay Cortez in Minneapolis at mcortez@bloomberg.net;Bailey Lipschultz in New York at blipschultz@bloomberg.net
To contact the editors responsible for this story: Drew Armstrong at darmstrong17@bloomberg.net, Cécile Daurat, Mark Schoifet
©2019 Bloomberg L.P.