Iovance and Amgen Are Making Cancer History
Iovance and Amgen Are Making Cancer History
(Bloomberg Opinion) -- The annual meeting of the American Society of Clinical Oncology (ASCO), which wrapped up Tuesday, is one of the drug industry’s most prominent platforms for showcasing promising cancer research and treatments. This year was no exception.
While there wasn’t as much late-stage blockbuster data to pore over as in years past, there was plenty of exciting progress to celebrate among efforts to tackle tough cancers. Early results from Iovance Biotherapeutics Inc. in cervical cancer and Amgen Inc. in lung cancer were particular standouts, and broke new scientific ground.
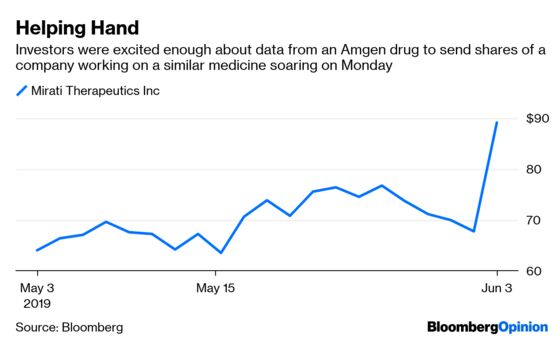
Iovance shares have climbed more than 50 percent since its data was first released in mid-May, including a 10 percent surge on Monday (the company presented at the Chicago conference on Friday). Amgen shares climbed more than 4 percent this week, while Mirati Therapeteutics Inc. – a smaller rival that’s working on a similar lung cancer drug to Amgen’s surged more than 45 percent in that same time.
It’s easy to over-hype initial results, and there have been many instances where thrilling early data and a ballooning stock price deteriorated over time. In the case of Iovance and Amgen, though, the excitement is warranted, and the impact should extend beyond a few amped-up investors.
Iovance’s treatments involve isolating a specific type of cancer-fighting immune cell from individual patients, then generating millions of copies of them and injecting them back into cancer sufferers. A similar approach has resulted in the approval of two potentially curative blood-cancer drugs, but applying it to solid tumors in other parts of the body at any kind of scale has been a much slower process. Iovance appears to have broken through that barrier with its so-called TIL therapies. In the updated data presented at ASCO, the treatment helped 44% of a group of heavily pre-treated cervical-cancer patients who would otherwise have little in the way of useful options.
Amgen also did something exceedingly difficult in successfully targeting KRAS, a protein which is common to several cancers but was previously considered “undruggable.” Half of a group of 10 lung-cancer patients in the trial saw their tumors shrink as of the firm’s ASCO presentation, an improvement on earlier data.
As for many drugs in development, caveats abound for both treatments. More time and much bigger trials are needed, and a related Iovance cell therapy and Amgen’s drug weren’t quite as impressive in melanoma and colorectal cancer, respectively. Also, bespoke cell therapies including Iovance’s are costly, have side effects, and require difficult pre-treatment regimens which limit their commercial appeal. But in an industry where it’s too common for companies to replicate the successes of others, these potentially groundbreaking advances are notable.
Extending cell therapy into solid tumors is a huge step forward. Iovance’s treatment is already on its way to being an option for patients with nowhere else to turn. In the best-case scenario, the company’s success will attract more investment toward a problem that scared off many researchers before, and its approach will be refined and improved over time. As for Amgen, its results will boost companies working on related treatments, and its treatment is already pegged as a potential blockbuster.
The world of cancer research needs more exciting ideas to chase. Let’s hope these early successes by Iovance and Amgen hearten and encourage others in the field to attempt even more quixotic feats of medical chemistry and biology.
To contact the editor responsible for this story: Beth Williams at bewilliams@bloomberg.net
This column does not necessarily reflect the opinion of the editorial board or Bloomberg LP and its owners.
Max Nisen is a Bloomberg Opinion columnist covering biotech, pharma and health care. He previously wrote about management and corporate strategy for Quartz and Business Insider.
©2019 Bloomberg L.P.