Here’s What Happens Now That Oxford-AstraZeneca Vaccine Won U.K. Clearance
Here’s What Happens Now That Oxford-AstraZeneca Vaccine Won U.K. Clearance
(Bloomberg) -- The U.K. approved a second Covid-19 vaccine, and it’s a homegrown one this time. The government has ordered 100 million doses of the shot developed by AstraZeneca Plc and the University of Oxford, more than any other candidate.
When will the vaccination start?
The first doses are being released Wednesday and vaccination will start next Monday. AstraZeneca says it aims to supply millions of doses in the first quarter.
The priority should be to give as many people in at-risk groups their first dose rather than provide the required two doses in as short a time as possible, the government says. But people should get the second shot four to 12 weeks after the first. The first vaccine the U.K. approved, made by Pfizer Inc. and BioNTech SE, requires two injections three weeks apart.
How will I know when it’s my turn?
The National Health Service says it will contact people when it’s their turn to be vaccinated, and has emphasized it’s important not to reach out before then.
How does the vaccine differ from others?
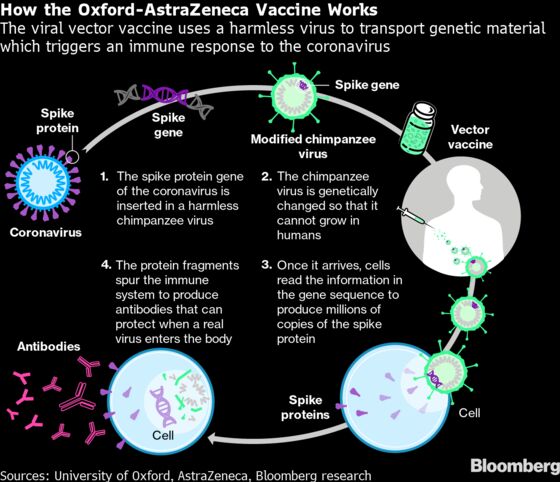
The product uses a harmless chimpanzee virus to transport genetic material that triggers an immune response to the coronavirus. That’s different from BioNTech and Pfizer’s messenger RNA approach, which transforms the body’s own cells into vaccine-making factories.
When patients were given two full doses, the Astra-Oxford vaccine was 62% effective in an advanced trial -- less than the Pfizer-BioNTech one and another from Moderna Inc. A small group that mistakenly received half of the first dose showed better protection, with efficacy reaching 90%. But participants were 55 years old or less, and because older people who are most at risk of severe Covid-19 often show more sluggish immune responses, the results leave some doubt as to whether the higher efficacy will stand up to further testing.
Which dosage will be used?
The U.K. will administer the vaccine in two full doses rather than the half-dose, full-dose regimen. AstraZeneca has said it plans more clinical research to find out whether the results of the half-dose group hold up.
Why did the U.K. order more doses of this vaccine?
The homegrown shot is easier to transport and store: It can last six months at refrigerator temperatures, whereas the Pfizer-BioNTech vaccine requires deep freezing. The majority of doses will also be produced locally, which should help avoid any supply delays.
Can I choose which vaccine I get?
Probably not. The important thing to remember is that both products protect against severe disease, which is the ultimate goal of the vaccination effort.
Can I get both vaccines?
A regulatory panel advised against mixing doses because a combination hasn’t been tested, but it’s possible shots could be pooled in the future if studies show that can produce an enhanced immune response.
The U.K.’s Vaccine Taskforce has outlined plans to test combinations of approved shots next year to see if a mix could boost immunity, the panel said this month. The first tests will combine the Pfizer and Astra vaccines.
A combination should work for vaccines that target the spike protein of SARS-CoV-2, according to Andrew Pollard, who led the University of Oxford’s vaccine trial with Astra. Both vaccines, as well as the Moderna one on sale in the U.S., use the spike protein as a target.
©2020 Bloomberg L.P.