FSD Pivots to Pharma From Pot During Pandemic: Cannabis Weekly
FSD Pivots to Pharma From Pot During Pandemic: Cannabis Weekly
(Bloomberg) -- Talk about a well-timed pivot.
FSD Pharma Inc., a Canadian company that began life as a cannabis cultivator with the ticker HUGE, announced June 3 that it received U.S. Food and Drug Administration approval to design a proof-of-concept study for a potential Covid-19 treatment, sending its shares up 131%.
The treatment doesn’t involve any sort of cannabis-derived compound. Instead, FSD is testing a naturally occurring fatty acid called ultramicronized PEA, which has been shown to help with inflammation. FSD acquired the worldwide rights excluding Italy and Spain to ultramicronized PEA from Epitech Group about a year ago for $17.5 million, according to Chief Executive Officer Raza Bokhari.
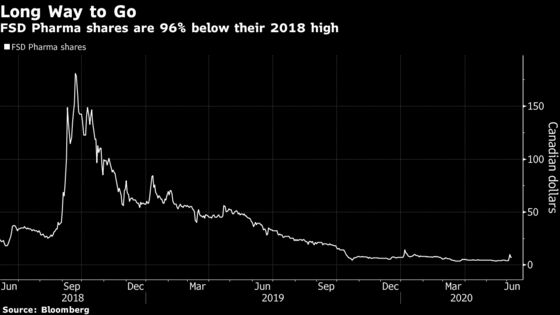
That was part of a broader shift at the company toward research and development into synthetic compounds that target the endocannabinoid system of the human body. While it still has a cannabis cultivation facility in Cobourg, Ontario, that part of the business has been “scaled down to a bare minimum” amid the pandemic, Bokhari said in a phone interview.
“Our ongoing view is to hunker down on our cannabis grow efforts and double down on our biosciences and specialty R&D pharmaceutical efforts,” he said.
FSD contacted the FDA in late March after it heard Italian physicians and scientists were advocating the use of ultramicronized PEA as a Covid-19 treatment. “The FDA has been, I must say, very responsive and very prompt in responding, and they now have granted us permission to move ahead” with designing a Phase 2a clinical trial, Bokhari said. “They’re working at lightning speed.”
The company is actively searching for another “compelling synthetic compound that would fall in our scope of expansion,” Bokhari said. However, he remains skeptical of claims that cannabis-derived compounds such as CBD can help treat Covid-19.
“I don’t intend to trivialize any potential role that molecule can offer, but without rigorous clinical trials and FDA-approved guidelines, I believe there are more claims out there than can actually deliver results,” he said.
The FDA has issued warning letters to firms selling fraudulent products that claim to treat Covid, including a company called CBD Gaze that advertised “the best CBD oil to help fight coronavirus.”
Events This Week
MONDAY 6/8
- Stifel’s virtual Cross Sector Insight Conference features presentations from several cannabis companies (through June 10)
TUESDAY 6/9
- The Central European Cannabis Forum holds a virtual conference (through June 10)
WEDNESDAY 6/10
- GW Pharmaceuticals Plc presents at Goldman Sachs’s virtual Global Healthcare Conference
- Neptune Wellness Solutions Inc. will report results postmarket
THURSDAY 6/11
- Hexo Corp. reports earnings premarket
- Tilt Holdings Inc. reports postmarket
Last Week’s Top Stories
Canopy Gets Four Downgrades Amid Slow Turnaround and High Costs
Tilray Lockup Expiring June 11 as Stock Outperforms Benchmark
©2020 Bloomberg L.P.