Crispr Gene Editing Shows Promise in Blood Disease Study Updates
Crispr Gene Editing Shows Promise in Blood Disease Study Updates
(Bloomberg) -- Updated results from a first-in-human study using the gene-editing tool Crispr showcased the promise for a company named after the technology and its partner Vertex Pharmaceuticals Inc.
Crispr Therapeutics AG and Vertex said a total of 10 patients, seven with beta thalassemia and three with severe sickle cell disease, saw benefits after receiving their one-time treatment CTX001. After three to 18 months of follow-up, all of the patients remained free from a once-routine need for blood transfusions and were rid of painful events related to their diseases.
Investors who have been awaiting the update will get a chance to see results in a presentation at the virtual American Society of Hematology (ASH) meeting as well as an article in the New England Journal of Medicine. ASH is the year’s largest medical meeting on blood diseases and disorders and will feature updates from competing medicines in development.
“The results are consistent and they are durable,” said David Altshuler, Vertex’s chief scientific officer, highlighting that the pace of enrolling new patients in the programs has been ramping up quickly. “This is the first publication of Crispr used to treat inherited disease in human to our knowledge,” he said in a phone interview, referencing the NEJM article.
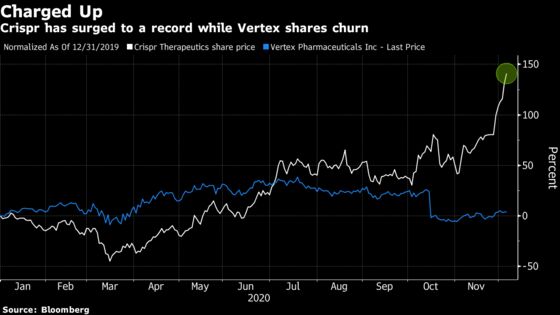
Shares of Crispr and Vertex have gone different ways in 2020, especially after Vertex scrapped development of another medicine dubbed VX-814 in mid-October, prompting a selloff. While Crispr shares have soared 140% to a string of record highs amid optimism for the company’s platform, Vertex shares are up less than 5% year-to-date.
The CTX001 data are likely to benefit companies such as Intellia Therapeutics Inc. and Editas Medicine Inc. that are also studying medicines based on Crispr gene-editing. And the sickle cell findings may be compared with fresh data from Bluebird Bio Inc.’s gene therapy LentiGlobin, which will be presented at the ASH conference Monday evening. Optimistic Bluebird analysts expect continued durability of the therapy to help push shares higher as both programs advance.
The CTX001 therapy’s safety was generally consistent with other treatments needing chemotherapy to blast a patient’s immune system. Among beta thalassemia patients there were four serious adverse events reported in one patient including headache, hemophagocytic lymphohistiocytosis (HLH), acute respiratory distress syndrome and idiopathic pneumonia syndrome. The issues eventually were resolved and there were no serious adverse events deemed related to the treatment for the sickle cell patients.
“We think it’s probably a one-off and we’re unlikely to see it in more patients, but it’s something we’ll observe carefully,” Crispr Therapeutics Chief Executive Officer Samarth Kulkarni said discussing the HLH event, a potentially fatal systemic inflammatory syndrome.
Transfusion Free
The beta thalassemia patients had hemoglobin between 9.7 and 14.1 grams per deciliter, compared with a range of 12 to 17 in healthy adults. They also had fetal hemoglobin between 40.9% and 97.7%, further showing the therapy’s benefit.
“The most important endpoint is whether these patients are transfusion free and all of these patients are,” said Vertex’s Bastiano Sanna, chief of cell and genetic therapies. “That is the important endpoint and what we are tracking for these beta thalassemia patients.”
The three sickle cell patients remained free of painful complications known as vaso-occlusive crises, which require hospitalization, and had total hemoglobin levels between 11.5 and 13.2 grams per deciliter -- of which 31.3% to 48% was fetal hemoglobin.
The therapy works by editing genes found inside blood stem cells that affect the production of hemoglobin made by newborn babies. The natural process is good for most people, allowing the body to start making adult hemoglobin. For patients with beta thalassemia and sickle cell disease, however, their adult hemoglobin is flawed.
Doctors extract the patients’ stem cells and send them to a manufacturing facility where they are edited. The patients are given chemotherapy to wipe out their immune systems and make room for the transformed cells. The edited cells are then infused back into the patients.
©2020 Bloomberg L.P.