Crispr Surges as Gene Editing Shows Promise in Blood Disease
Crispr Gene Editing Shows Early Promise in Blood Disease Study
(Bloomberg) -- A first-in-human study using the gene-editing tool Crispr has a company named after the technology and its partner, Vertex Pharmaceuticals Inc., claiming an early victory in treating two inherited blood disorders.
Crispr Therapeutics AG and Vertex said a pair of patients -- one suffering from beta thalassemia and the other with severe sickle cell disease -- saw benefits from one-time treatment with CTX001. While they’ve been followed for just nine months and four months, respectively, their painful flare-ups and once-routine need for blood transfusions stopped after getting the therapy. Both developed severe complications including infections that were deemed unrelated to the gene-editing treatment.
Investors have been awaiting the results and many expected to hear them at the American Society of Hematology meeting in Orlando next month, where reports on three patients with advanced cancers treated with a similar approach at the University of Pennsylvania will be presented. The companies elected instead to get data from more patients and present the findings at a medical meeting next year, said Crispr Therapeutics Chief Executive Officer Samarth Kulkarni.
“While the data are early, we are quite excited about what we are seeing,” he said in a telephone interview. “This is a pretty significant milestone, not just for us as a company but for the entire field. This could be an important landmark in medicine, when we saw the first promise for providing cures for a number of diseases using a gene editing approach.”
The early findings may benefit rival companies also studying medicines based on Crispr technology, as they are the first results from publicly traded companies using the platform. Editas Medicine Inc.’s lead drug will be given to its first patient at the start of next year as a treatment for a form of blindness, while Intellia Therapeutics Inc. is on track to file for its first human trial by mid-year.
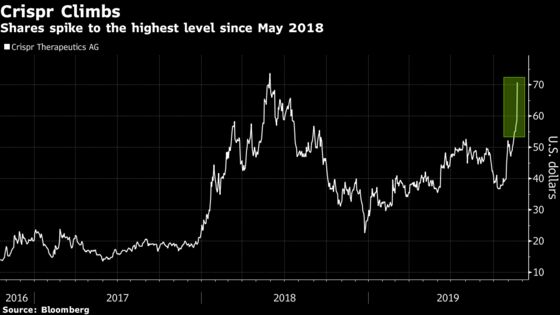
Crispr shares surged 21% at 9:40 a.m. in New York to the highest since May 2018 as Vertex rose to another record high. Intellia shares jumped 17%, the most since August 2017 while Editas climbed 13% for its best session since March.
The results may put investors at ease because the level of hemoglobin, an oxygen-carrying protein found in red blood cells, rose above 11 grams per deciliter to near-normal levels in both patients. The therapy also stimulated production of fetal hemoglobin, a form that doesn’t carry the damaging characteristics found in adults with both diseases, a sign of the approach’s effectiveness.
Read more: Crispr Upgraded at Oppenheimer Before First-In-Human Data
The treatment works by editing genes found inside blood stem cells that suppress the production of hemoglobin made by newborn babies. The natural process is good for most people, allowing the body to start making adult hemoglobin. For patients with beta thalassemia and sickle cell disease, however, their adult hemoglobin is flawed.
The researchers extracted the patients’ own blood stem cells and sent them to a manufacturing facility. There, they were edited to reverse the process that switches hemoglobin production from the fetal form to the flawed adult form. The patients were given strong chemotherapy to wipe out their immune systems, a necessary step to make room for the transformed cells. The gene-edited cells were infused back into the patients, where they took hold and started producing healthy fetal hemoglobin.
In the beta thalassemia patient, total hemoglobin levels were 11.9 grams per deciliter, with 10.1 grams per deciliter being fetal hemoglobin. Almost all cells expressed fetal hemoglobin. The sickle cell patient was free from painful events and had hemoglobin of 11.3 grams per deciliter, with 47% being fetal hemoglobin.
While the beta thalassemia patient required 16.5 transfusions per year before the study, the patient was able to safely give blood after the treatment, David Altshuler, Vertex’s chief scientific officer, said. “The person’s hemoglobin is rock stable,” he said.
Both patients suffered severe adverse events during treatment. The beta thalassemia patient had pneumonia and a liver complication, while the sickle cell patient had a severe infection, gallstones and abdominal pain. The issues were caused by the chemotherapy rather than the gene-editing treatment, and they all eventually resolved.
“One of the important milestones was safety,” Vertex CEO Jeff Leiden said by phone. “The adverse events were generally mild to moderate and were all associated with the bone marrow conditioning and not with the product itself.”
The work on beta thalassemia and sickle cell disease, the most common inherited blood disorder in the world, are a welcome change after years of neglect. Novartis AG recently won U.S. approval for Adakveo, the first approved drug that specifically attacks sickle cell disease. In beta thalassemia, CTX001 would likely compete with Bluebird Bio Inc.’s gene therapy Zynteglo, which was approved in Europe this summer. Kulkarni said it’s too soon to compare treatments, though he believes gene editing is better.
“We view gene editing as a superior approach because you’re taking a scalpel and making a precise edit in the genome, and that should render the therapy safer than a gene therapy approach,” he said.
Vertex’s Leiden said the early data may herald dramatic future benefits.
“Crispr/Cas9 gene editing technology has real curative potential for serious genetic diseases,” he said. Vertex has been building a “gene-editing toolbox” that Leiden said he believes is validated by these early findings.
While the companies gave data on only two patients, they have enrolled several others and are picking up the pace of treatment, Kulkarni said. The results give them confidence in their ability to move faster, enroll patients at a number of different medical facilities and dose patients in parallel, he said.
Crispr is also studying medicines in both solid and blood cancers and is in the early stages of developing treatments for diabetes and cystic fibrosis.
To contact the reporters on this story: Bailey Lipschultz in New York at blipschultz@bloomberg.net;Michelle Fay Cortez in Minneapolis at mcortez@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Will Daley
©2019 Bloomberg L.P.