Bristol Myers CVR Falls as FDA Jitters Overshadow Huge Potential
Bristol Myers CVR Falls as FDA Jitters Overshadow Huge Potential
(Bloomberg) -- Bristol Myers Squibb Co.’s comments that U.S. regulators still haven’t planned to inspect a facility that makes one of its experimental cancer drugs overshadowed news that a filing for another one of its cancer treatments with Bluebird Bio Inc. was accepted for review by the Food and Drug Administration.
The so-called contingent value right, or CVR, that depends on both drugs gaining approval fell as much as 9.1% on Tuesday on more than five-times the average volume.
The $9 all-or-nothing CVR, which Bristol Myers used to sweeten its purchase of Celgene Corp., depends on timely approval of the first drug liso-cel by the end of the year and second Bluebird Bio drug ide-cel by March 31.
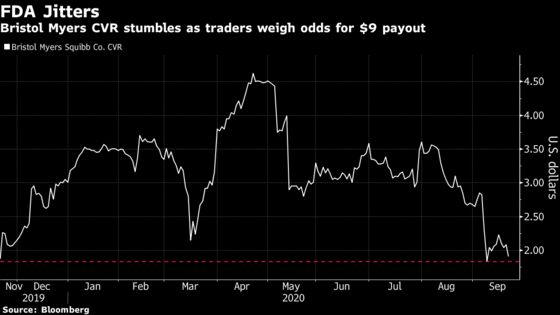
Bristol Myers’s comments that there are no dates set up for FDA inspection of the two facilities related to liso-cel are “borderline” confusing given the lack of clarity, Mizuho analyst Salim Syed wrote in a note. While “nobody is expecting” Bristol Myers to have a confirmed date for the inspection, the lack of a tentative date “is a bit perplexing here to some folks,” he wrote.
The CVR sank earlier this month when management at Citi’s BioPharma Virtual Conference said the site inspections for the cell-therapy facilities related to liso-cel weren’t inspected. A decision from the FDA on the drug as a treatment of adults with relapsed or refractory large B-cell lymphoma is expected by Nov. 16.
Meanwhile, the acceptance of Bristol Myers and Bluebird’s filing is a piece of good news for the derivative, and is worth about a quarter to the CVR, Syed wrote. A targeted decision by March 27 would allow the approval to sneak by ahead of the March 31 deadline.
Bluebird analysts were more optimistic at first blush despite shares being little changed on Tuesday. Approval is expected and the companies remain on track to capitalize on a first-mover advantage ahead of competition from Johnson & Johnson and partner Legend Biotech Corp., wrote RBC analyst Luca Issi.
©2020 Bloomberg L.P.