Biogen Slips as Roche Spikes Alzheimer's Trials on Weak Data
Biogen Slips as Roche Spikes Alzheimer's Trials on Weak Results
(Bloomberg) -- Shares of Biogen Inc. came under pressure in trading before the U.S. market opened Wednesday after peer Roche Holding AG halted a pair of studies in Alzheimer’s disease as early data hinted that its drug wasn’t working.
The news is another blow for drugmakers studying treatments of the disease and prompted investors to weigh the impact Roche’s failure will have on late-stage tests of Biogen’s closely watched treatment, aducanumab. Cantor Fitzgerald analyst Alethia Young wrote that the read-across to aducanumab is limited, however both medicines belong to the same class of drugs.
“Biogen noted on their call yesterday morning that they would be cautious of a read-through,” Young wrote in a note to clients, “but we are sure investors will debate this since the drugs are the same class.”
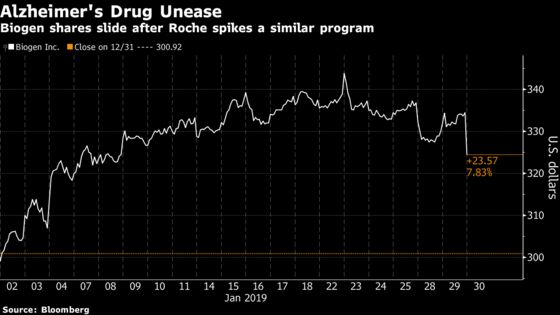
Shares of the Cambridge, Massachusetts-based drugmaker fell 3.2 percent at 10:00 a.m. in New York, the most in a month. Roche’s stock was little changed in Switzerland while its partner AC Immune SA plummeted as much as 70 percent to an all-time low.
Investors remain zeroed in on the progressing results from Biogen’s final-stage tests of aducanumab, which finished enrolling patients over the summer. The timing the data has yet to be solidified, but analysts have speculated that the final results will come in the beginning of 2020, with a first peek potentially coming in the first half of this year.
Here’s what some analysts are saying:
Stifel, Paul Matteis
“The failure of crenezumab offers a blow to the (evolved version) of the amyloid hypothesis, though its hard to quantify the exact readthrough onto aducanumab without seeing/understanding the detailed data.”
“There are very meaningful differences between crenezumab and aducanumab” though the failure can’t be entirely dismissed because both companies are targeting a similar population and employed the same endpoint.
“We still think aducanumab still has a real shot (unlike cren, its phase-Ib data are best-in-category) -- but this news weakens the case, even if just a little.”
Rates buy with a $397 price target
Cantor Fitzgerald, Alethia Young
Aducanumab has shown statistically significant efficacy in a Phase 1 trial whereas Roche’s crenezumab did not. While aducanumab and crenezumab are similar compounds they are “structurally very different amyloid beta antibodies,” which may bode well for Biogen.
“Biogen noted on their call yesterday morning that they would be cautious of a read-through, but we are sure investors will debate this since the drugs are the same class.” She maintains estimates and probability of success for aducanumab.
Rates overweight with a $400 price target.
SVB Leerink, Geoffrey Porges
“We expect downward pressure on Biogen’s stock today as a result of this disclosure based on investors’ potential read-through to the outcome of the aducanumab phase III program. However, we do point out that crenezumab was a higher risk asset and has not shown the potential to reduce amyloid plaque burden in prior studies.”
Rates shares market perform
Jefferies, Michael Yee
There are key differences between the medicines as Biogen has “clearly cautioned not to read too much into” any comparisons as crenezumab is “(1) different antibody w/ lack of effector function, (2) doesn’t cause ARIA even at higher doses (possibly due to on-target not removing plaque), (3) binds oligomers whereas BIIB targets more insoluble aggregated plaque.”
Rates Biogen as a hold with a $380 price target
To contact the reporter on this story: Bailey Lipschultz in New York at blipschultz@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Cristin Flanagan
©2019 Bloomberg L.P.