Biogen’s 2022 Boom or Bust Hangs on Alzheimer’s Therapy
Biogen’s 2022 Boom or Bust Hangs on Alzheimer’s Therapy
(Bloomberg) -- With some 1,800 of its 3 million members diagnosed with memory-robbing Alzheimer’s disease, Blue Cross Blue Shield of Massachusetts desperately wants to provide an effective treatment. But even when Biogen Inc. cut the price of Aduhelm in half, the insurer still refused to cover it.
“Our decision did not have anything to do with the price,” said Chief Medical Officer Sandhya Rao. “It was more around the lack of solid data on effectiveness of the drug.”
After a year of excitement over a treatment approved to attack Alzheimer’s, Biogen faces a critical 2022 as U.S. health programs, insurers, doctors and patients raise or lower their thumbs on Aduhelm. The company already plans to cut costs by $500 million in 2022 as it evaluates its best path to growth.
Potential acquirers may sense weakness: Korea Economic Daily reported Wednesday that Samsung Group is in talks to purchase the Cambridge, Massachusetts-based biotech. A representative for Biogen said that the company doesn’t comment on market rumors and speculation. Samsung Biologics Co., a biotech unit of the Samsung Group, said in a regulatory filing that the story isn’t true. In the midst of a long-awaited turnaround, there’s only one story worth watching at Biogen, said Cantor Fitzgerald health-care analyst Alethia Young.
“Aduhelm,” she said. “That’s all it is.”
Biogen shares fell 6.6% in premarket trading Thursday. They had surged as much 9.5% Wednesday in New York after the Korean report.
In Aduhelm, analysts and investors saw a potential windfall, with annual sales estimated as high as $5 billion ushering in a new era of growth after years of uncertainty. Instead, the drug’s approval ignited a firestorm of controversy, leaving behind widespread skepticism about its ability to slow the progression of Alzheimer’s.
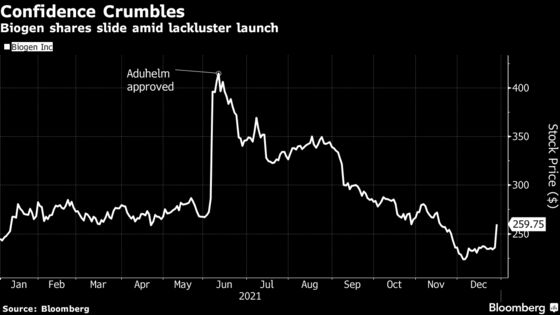
Aduhelm has been shown to remove amyloid plaque, a hallmark of Alzheimer’s disease, from the brain; that was the justification for its approval. But there’s no proof from patient brain-function tests that Aduhelm slows the memory-wasting condition, and Biogen’s own studies on that score have yielded contradicting results.
Still, Biogen saw a product that could replenish revenue from its fading multiple sclerosis franchise that includes blockbusters like Tecfidera. Alzheimer’s, the cause of dementia in about 6.2 million Americans, was a worthy target. Other items in the portfolio, like the Spinraza therapy for a childhood muscle condition, offered far less opportunity.
“The potential value creation -- or destruction -- for other assets they have pale in comparison to Alzheimer’s,” said Jefferies & Co analyst Michael Yee.
Coverage Decision
The company reported just $300,000 in Aduhelm sales in the third quarter, although it estimates 50,000 people will start infusions in 2022. Regulators in Europe and Japan said they need more evidence before they can approve the drug, handing Biogen two more setbacks.
A critical point is slated for January when Biogen’s most important customer, the U.S. Medicare program, shares its plan for covering the drug and similar ones. Medicare is set to determine who among its roughly 63 million beneficiaries -- mainly older people who constitute the vast majority of potential patients -- can receive Aduhelm.
A final decision is expected in April. Private insurers are likely to follow the federal health program’s lead, and a clear path to reimbursement could open a major bottleneck in Aduhelm’s uptake.

Among the reasons for hesitation on the drug is its Food and Drug Administration approval under an accelerated pathway that uses a looser standard than the traditional route. Biogen upped the ante with a $56,000 price tag for a year’s treatment, a lofty figure that some analysts predicted could decimate budgets. Medicare hiked premiums for seniors even before CMS decides whether to cover Aduhelm because of cost fears.
The company has since cut the price of Aduhelm in half. But it still costs twice as much as analysts anticipated before it hit the market, said Brian Abrahams, a biotech analyst at RBC Capital Markets.
“It was a clear strategic blunder – especially after the accelerated approval – to price at that level,” Abrahams said.
Adding Evidence
The conflicting results on patient cognition are another major reason insurers aren’t rushing to cover the drug.
“The lowered price does not change this lack of evidence,” Stefanie Park, assistant vice president of medical management at the Hawaii Medical Service Association insurer, said in a statement.
The doubts could prove tough to overcome -- at least right away. Biogen plans to start screening patients in May for another trial of Aduhelm. But results won’t be available for at least 18 months after the participants are treated.
Competition Creep
That may give other Alzheimer’s drugs on the horizon more time to show whether they can outdo Aduhelm. One of those belongs to Biogen itself and partner Eisai Co Ltd., which plan to share trial results from the drug, lecanemab, in the second half of 2022. Positive results may bolster support for Aduhelm, though a negative outcome will only deepen skepticism around the entire class of plaque-removing drugs, Abrahams said.
Meanwhile, Eli Lilly & Co. hopes to launch its Alzheimer’s treatment, donanemab, next year, if it’s approved. Roche Holding AG expects late-stage trial data from its experimental therapy gantenerumab in 2022.
Even if other therapies are proven to work, Biogen will still need to change the perception around Aduhelm if it hopes to turn the product into a success. That’s a daunting ask for any drugmaker.
“Some people say you only get one chance to launch a drug,” Cantor’s Young said.
And Biogen may have already had its chance.
©2021 Bloomberg L.P.