AbbVie Is Losing Its Humira Safety Blanket
AbbVie Is Losing Its Humira Safety Blanket
(Bloomberg Opinion) -- AbbVie Inc. has a pretty sunny outlook for a company facing the mortality of its key product.
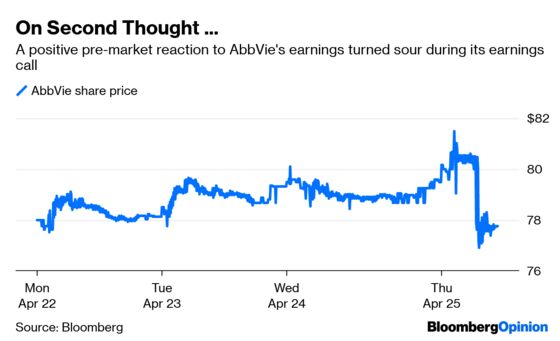
The company reported on Thursday that first-quarter sales of its blockbuster inflammation drug Humira fell 5.6 percent from the same period last year. The drug had not posted a year-over-year sales decline in at least eight years.
AbbVie was still able to increase its 2019 earnings guidance and consistently projects confidence about its ability to replace a medicine that accounts for more than 60 percent of its sales. But with Humira sales declining in Europe because of competition from cheaper copies and slowing in the U.S., AbbVie’s upside case depends on the company striking drug industry gold several more times.
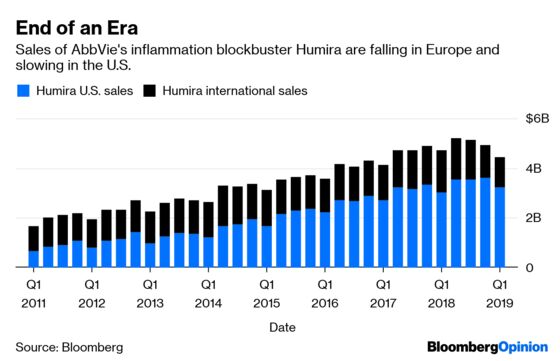
AbbVie’s two most important new drugs are successor medicines for Humira, which will most likely face serious biosimilar competition in the larger U.S. market in 2023.
The first is Skyrizi, initially approved to treat psoriasis on Tuesday. The second is upadacitinib, which is likely to be approved for rheumatoid arthritis patients in the next year. AbbVie thinks these drugs will win approval for other conditions and combine to generate more than $10 billion in “incremental risk-adjusted sales” by 2025. This initial approval will put that prediction to the test.
Neither drug is first-in-class; AbbVie is betting that they’ll overcome that by being the best. It wants to reprise the success of third-to-market Humira, which ended up dwarfing the sales of speedier rivals.
AbbVie’s new medicines have produced good data, and the company has excellent and long-lasting relationships with the physicians that will be prescribing them. But even with that pedigree, the drugmaker will have a hard time hitting its targets and keeping shareholders happy.
Both of these drugs could cannibalize Humira sales, potentially accelerating its decline. The inflammation drug market is more competitive now than it has ever been. Skyrizi and upadacitinib will compete with multiple established and recently approved new options in just about every area they’re approved in, as well as cheaper biosimilars in Humira’s class.
Insurers and pharmacy benefit managers have become better and more aggressive at stonewalling the introductions of costly new medicines and creating pricing pressure in the more than 15 years Humira has been on the market, which will make it more difficult for AbbVie’s new drugs to catch up. The company also can’t take big price increases in the current political environment, and more restrictive drug pricing policy has growing bipartisan support.
AbbVie’s big expectations for its next generation of products are now going to have to prove out in the real world. Without the cushion of Humira’s endless growth, any failure to measure up is going to be judged even more harshly.
To contact the editor responsible for this story: Daniel Niemi at dniemi1@bloomberg.net
This column does not necessarily reflect the opinion of the editorial board or Bloomberg LP and its owners.
Max Nisen is a Bloomberg Opinion columnist covering biotech, pharma and health care. He previously wrote about management and corporate strategy for Quartz and Business Insider.
©2019 Bloomberg L.P.