Consider This a Warning on Cancer-Drug Combos
Consider This a Warning on Cancer-Drug Combos
(Bloomberg Opinion) -- The pharmaceutical industry believes that the lucrative future of cancer treatment lies in combining immune-boosting medicines with other drugs to boost their effectiveness. But that future is still a ways off, and the present is pretty ugly.
It’s something to keep in mind as one of the year’s biggest cancer conferences gets underway in Chicago.
The backbones of most cancer combo trials are a group of successful drugs called PD1/L1 inhibitors — led by Merck & Co.’s Keytruda and Bristol-Myers Squibb & Co’s Opdivo — that help unleash the human immune system against cancer. While these medicines can have a dramatic impact on some patients, they don't work for most. The hope is that combining them with other treatments might make them work better and enable further expansion into more cancers.
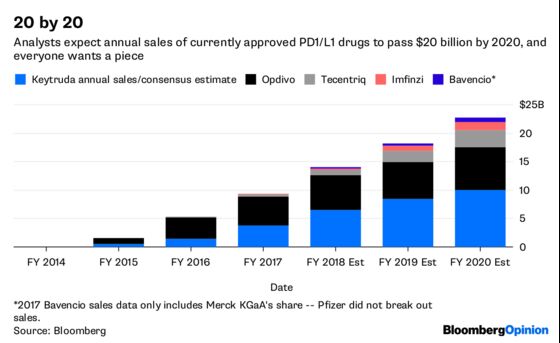
The appeal of combos is clear and there are already five of these medicines on the market, with more are on the way. As drugmakers fight to get an edge, they are running an incredible number of combination trials. Merck and Bristol-Myers alone are involved in more than 500, according to Bloomberg Intelligence. No wonder, then, that new data on these treatments will be a highlight at this year’s annual meeting of the American Society of Clinical Oncology (ASCO), which began Friday and runs through June 5.
Investors need to remember, though, that up to now the hype over combos has dramatically outpaced delivery. Just last month, Genmab A/S revealed that its partner Johnson & Johnson was halting trials combining its drug Darzalex with PD1/L1s due to safety and efficacy issues. Genmab’s shares went on to drop more than 20 percent. And that’s only the latest disappointment.
Bristol got the combination of Opdivo and its older immune-boosting drug Yervoy approved for melanoma back in 2015, but uptake has been sluggish, in part because the second drug adds side effects. A kidney cancer approval may boost sales, but the combo has badly disappointed in lung cancer – the biggest market available. AstraZeneca PLC’s heavy investment in a similar approach has yielded little so far.
Perhaps the most glaring example of the promise and pitfalls of these combos is Incyte Corp.’s epacadostat. Very early data from a combination trial with Keytruda helped boost Incyte’s market cap to $30 billion last year. Companies scrambled to start trials with the drug or similar medicines in a variety of cancers. The drug failed to show any benefit whatsoever in its first final stage trial, leaving a sizeable chunk of the industry with egg on its face. The firm is now worth $14.2 billion.
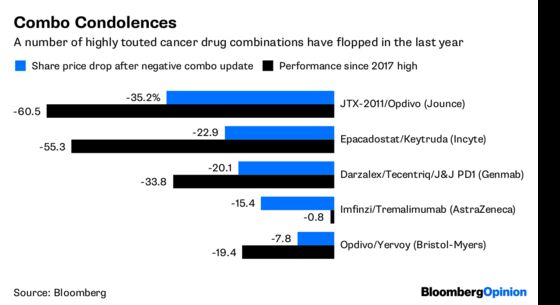
As ASCO continues, we are likely to see stocks plunge and soar on newly revealed data. But caution is warranted. All of the data that’s going to emerge is from trials that began well before the recent spate of failures. More setbacks are coming, and some of them will be from combos that look like successes this weekend.
Too many combination trials are based more on hope than solid scientific rationale. Many come from a fear of missing out or ceding a market to a competitor. In other cases, drugmakers have succumbed to the seduction of the economic advantages of owning both medicines in a successful combo. And decisions based on overreaction to small and difficult-to-interpret trials are rampant.
Too few take into account the high bar combos will have to clear to convince insurers to pay for two expensive drugs, and patients to accept additional side effects. Drugs like Keytruda don't have the same negative impact as traditional chemotherapy, but are by no means benign. Darzalex isn’t the first combo partner to add more safety issues, and it won’t be the last.
Eventually, drugmakers will learn from their mistakes and course-correct. But we’re likely to see the exploded fruit of a lot more bad decisions first.
To contact the editor responsible for this story: Beth Williams at bewilliams@bloomberg.net
©2018 Bloomberg L.P.