Moderna’s Post-Pandemic Chapter Hangs on Yet-to-Be Proven Drugs
Moderna’s Post-Pandemic Chapter Hangs on Yet-to-Be Proven Drugs
(Bloomberg) -- Wall Street has soured on Moderna Inc. -- one of 2021’s top performers on its ground-breaking Covid-19 vaccine.
Concerns were on display on Friday when the stock sold off as much as 14% after disappointing trial results from its experimental flu shot, which uses the same messenger-RNA technology as its coronavirus vaccine. Analyst price targets suggest about a 6% gain for the stock in the next 12 months -- after a 146% surge this year -- despite a pipeline of other drugs also using the same technology.
“There’s no margin for error,” said Julia Angeles a portfolio manager with Baillie Gifford who co-manages over $71 billion in assets. “As soon as there’s any deviation, there’s an overreaction,” she said. Baillie Gifford is the biggest holder in Moderna, with a roughly 10% stake as of Sept. 30, according to Bloomberg data.
Representatives for Moderna declined to comment.
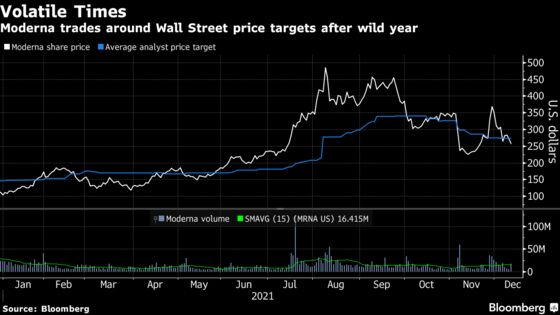
After touching a high of $484.47 in August, the Cambridge, Massachusetts drugmaker ceded its top spot as S&P 500’s best performer last month as the outlook for sales of its Covid shot weakened. Promising results for oral antivirals from Pfizer Inc. as well as Merck & Co. and an ongoing patent dispute on the Covid vaccine with the U.S. government have also weighed on shares.
Moderna gets the bulk of its sales from its Covid vaccine, increasing the importance of developing new therapies like the seasonal flu shot and its personalized cancer vaccine.
Wild Swings
Dubbed the Tesla of biotech earlier this year, Moderna was one of the most volatile stocks to trade on the S&P 500 Index this week, when a measure of 10-day volatility was more than five-fold greater than the average for the S&P 500 Index on Thursday.
More results from Moderna and Pfizer against the novel variant are expected in coming days and questions remain over the depth and breadth of the market for boosters in the years to come.
In a testament to the growing skepticism, Moderna is valued at 10 times estimated earnings, below the average of 12.2 for biotech peers in the index.
“It’s not just about next year, which may still be volatile,” Angeles said in an interview. “You need to believe in this opportunity for a new class of medicine.” The two funds co-managed by Angeles have beaten at least 95% of peers over the past three years, according to data compiled by Bloomberg.
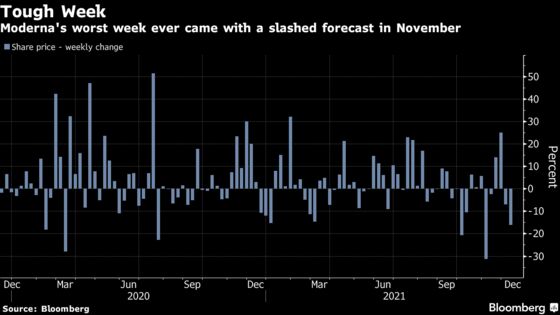
A study directly comparing Moderna’s flu vaccine to Sanofi’s already proven one would likely be needed, said Bloomberg Intelligence’s Sam Fazeli after the results, “it shows how tough life can be post Covid in the much more competitive real world of vaccines.”
For Moderna’s flu shot, “there is a long way to go,” Fazeli said.
Moving Fast
Moderna pointed to the speed with which they’ve developed their flu shot and the potential to deliver a combination flu and Covid vaccine.
“Our first shot on flu is non-inferior to the best product on the market that people have worked on for 20 or 30 or 40 years in traditional pharma. So we’re going to keep moving very fast,” said CEO Stephane Bancel during a conference call discussing the results.
Other Moderna updates to watch for include an AstraZeneca Plc-partnered medicine for heart failure that had early-stage results in November, a vaccine for treating the deadly skin cancer melanoma and an inoculation for respiratory syncytial virus, a common infection in children that can lead to hospitalization.
After Moderna’s success against Covid-19, investors are looking for where else its technology can beat illness, says Nina Deka, a senior research analyst at Robo Global LLC, which holds Moderna in its exchange traded fund. “This is an opportunity to be game changing, it’s just a matter of when.”
©2021 Bloomberg L.P.