Crispr Gene Editing Shows Promise in Blood Disease Update
Crispr Gene Editing Shows More Promise in Blood Disease Update
(Bloomberg) -- Longer-term results from a first-in-human study of the gene-editing tool Crispr showcased the technology’s potential and delivered a win for the company that shares the platform’s name and its partner, Vertex Pharmaceuticals Inc.
Crispr Therapeutics AG and Vertex said a trio of patients, two with beta thalassemia and one with severe sickle cell disease, saw benefits from one-time treatment with CTX001. After a follow-up of five to 15 months, all three patients are free from their once-routine need for blood transfusions and painful events that came with their disorders.
“This is the first peer-reviewed, clinical evidence demonstrating that Crispr-Cas9-based gene editing does have curative potential for serious genetic diseases,” David Altshuler, Vertex’s chief scientific officer, said by telephone.
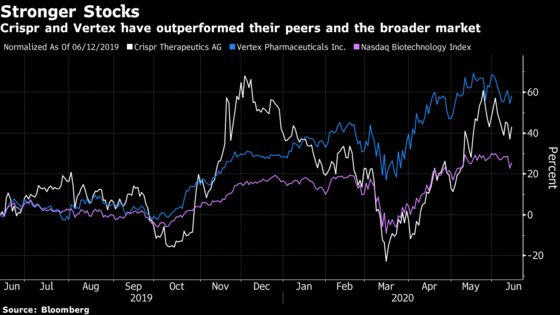
The highly anticipated results were showcased at the virtual European Hematology Association Annual Congress after an initial look at the results in November. Crispr shares rose as much as 6.1% in Friday’s session, the most in four weeks, while Vertex stock gained as much as 2.8%.
One beta thalassemia patient produced greater levels of hemoglobin, an oxygen-carrying protein found in red blood cells, as time went on through the 15 months of follow-up. The second patient with the disease was also producing normal levels of hemoglobin after five months. For both patients, the vast majority of what was produced was fetal hemoglobin, a form that doesn’t carry the damaging characteristics found in adults with the disease.
“To see the same level of hemoglobin expression and the same critical outcome when it comes to transfusion independence really shows that this is a proof of concept,” said Bastiano Sanna, Vertex’s chief of cell and genetic therapies. “It is unprecedented that this type of technology applied to this type of disease can show this type of result.”
The beta thalassemia patients had hemoglobin of 14.2 and 12.5 grams per deciliter, respectively, compared to a range of 12 to 15 normally seen in healthy adults.
The sickle cell patient remained free of painful complications known as vaso-occlusive crises, which require hospitalization, and had total hemoglobin levels of almost 12 grams per deciliter -- of which 46% was fetal hemoglobin.
“We have a lot more data in hand which gives us confidence that this can not only be a transformative effect for patients but can also be durable and be a one-time cure,” said Crispr Therapeutics Chief Executive Officer Samarth Kulkarni.
The treatment works by editing genes found inside blood stem cells that suppress the production of hemoglobin made by newborn babies. The natural process is good for most people and allows them to produce adult hemoglobin. However, that is not the case for patients with beta thalassemia and sickle cell disease who have flawed adult hemoglobin.
Doctors extract the patients’ blood stem cells and send them to a manufacturing facility where they are edited. The patients are given chemotherapy to wipe out their immune systems and make room for the transformed cells. The edited cells are then infused back into the patients.
All three patients in Vertex and Crispr’s testing had severe adverse events during treatment, though none were attributed to CTX001. The newest beta thalassemia patient had pneumonia and an upper respiratory tract infection, similar to what the other two saw previously. The issues eventually resolved.
Vertex is working to find more gentle ways to condition patients that do not have the same side effects as the blast of strong chemotherapy currently needed. So far, the companies have successfully treated five beta thalassemia patients with all of them producing mature red blood cells, a process known as engraftment. They have also dosed a second sickle cell patient and plan to provide additional data later this year.
For sickle cell patients, Novartis AG’s Adakveo and Global Blood Therapeutics Inc.’s Oxbryta are the only options, though neither treats the underlying disease. Bluebird Bio Inc.’s more advanced gene therapies, Zynteglo for beta thalassemia and LentiGlobin for sickle cell disease, have shown promise in both diseases and are likely to be CTX001’s main competition.
Both drug developers have outperformed the broader market and the closely watched Nasdaq Biotechnology Index over the past year. Crispr shares are up 41% over the past 12 months while Vertex has surged 58% compared to the Nasdaq Biotech Index’s 25% gain and a 6.8% boost for the S&P 500.
The study results provided a boost to others studying therapies based on Crispr gene-editing technology. Editas Medicine Inc. administered its lead drug for a form of blindness to the first patient earlier this year, while Intellia Therapeutics Inc. is on track to file for its first human trial by mid-year. Editas shares rose as much as 9.9%, the most intraday in a month, while Intellia gained 4.9%.
©2020 Bloomberg L.P.