Sage Shareholders Face Classic Biotech Investor Dilemma
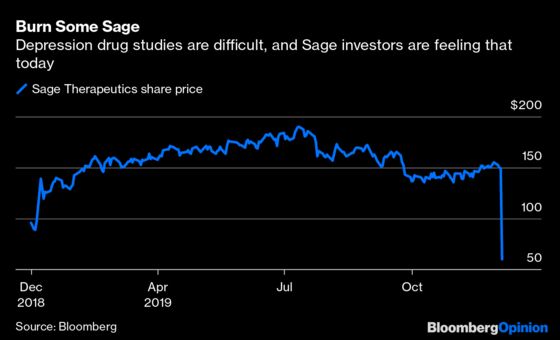
(Bloomberg Opinion) -- Investors in Sage Therapeutics Inc. woke up Thursday to every biotech investor’s nightmare. The company’s closely watched lead medicine, SAGE-217, failed to hit a key mark in a critical trial in patients with major depressive disorder. The stock was down a bruising 55% in early trading, cutting the firm’s market value by more than $4 billion.
Investors have a tough decision to make. They could join the many abandoning ship, assuming that the drug isn’t as effective as hoped and that additional continuing studies of the drug may fail. Or they can see this as a buying opportunity of an oversold stock. Sage’s executives spent a call with analysts Thursday outlining several reasons for optimism, and depression trials are notoriously difficult and fickle.
Eating a significant loss or betting on recovery will both take a strong stomach.
Drugs to treat depression almost always have a tough path to market. Patient improvement is difficult to assess, placebo effects are real and variable, and it’s hard to ensure drug compliance. Plenty of drugs have succeeded in mid-stage trials only to run into difficulty when tested in a larger population. Sage’s drug is both riskier and more promising because it takes a novel approach to treating the condition rapidly.
Sage has a few explanations for why the drug failed this study even though it succeeded in a late-stage trial in postpartum depression patients earlier this year. Some patients may not have taken the drug as directed or at all, which could have influenced results. The study also included a higher proportion of patients with less severe symptoms than previous tests.
Sage also pointed to the fact that the drug worked better at interim endpoints as evidence that the drug is active and that the study was “directionally” positive.
Optimistic investors may find this convincing. But there are plenty of valid reasons for the stock sell-off, too. The drug’s impact was substantially less pronounced than in previous studies, which may mean that it simply isn’t that potent and raises concerns that its effect may fade over time. Even compelling after-the-fact explanations of failed trials are somewhat unreliable. The company’s positive earlier trial in patients with major depressive disorder looked at a small number of patients; it may have exaggerated the drug’s impact. If the medicine does make it to market, it may treat a narrower group of patients than the company had once anticipated.
Sage could mount a comeback in a generally buoyant biotech market if future trials confirm that this failure was an unlucky fluke or if regulators are receptive to its various arguments. That’s a ride for only brave and patient investors; another failure would be received even less kindly than this one, and even good depression drugs are a gamble.
To contact the editor responsible for this story: Daniel Niemi at dniemi1@bloomberg.net
This column does not necessarily reflect the opinion of the editorial board or Bloomberg LP and its owners.
Max Nisen is a Bloomberg Opinion columnist covering biotech, pharma and health care. He previously wrote about management and corporate strategy for Quartz and Business Insider.
©2019 Bloomberg L.P.