Finally, Some Good News on Coronavirus Testing
Emergency approval of a new and faster coronavirus test from Roche is a positive, but more tools are needed.

(Bloomberg Opinion) -- The U.S. woke up to its first good coronavirus testing news in a while Friday, after weeks of hearing about nothing but delays and dangerously inadequate capacity.
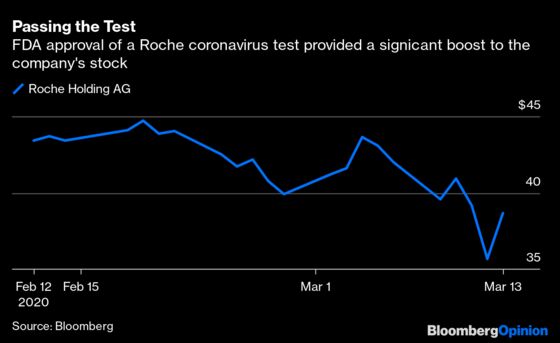
The Food and Drug Administration announced an emergency authorization of a Roche Holding AG test that can screen patients substantially faster than existing options. It's good news for Roche — the approval sent shares some 7% higher after a mostly down week — as well as for America's COVID-19 response. Once these tests are up and running, they should begin to help the U.S. catch up on a dangerous backlog and give more people the information and care they need.
Unfortunately, the country's inability to test enough people for weeks has contributed to an outbreak that is likely to grow substantially in the weeks to come and won't be contained any time soon. We don't just need more lab tests; other important tools we don’t have now — including so-called serologic tests and on-site diagnostics — could play a vital role in helping to better gauge the size of the outbreak and control its spread.
Serologic diagnostics allow extensive testing of samples from people who aren't confirmed COVID-19 cases. If people have been exposed and have developed antibodies against the virus, such tests will let public health officials know. This is incredibly valuable information in the fight against a disease that is mild or asymptomatic in many people. In addition to giving a better sense of how many cases we're missing and COVID-19's true fatality rate, it could also identify areas where it is spreading more quietly and help direct needed response. Centers for Disease Control director Robert Redfield recently told a Congressional committee that his agency has two tests of this type in development. Their speedy deployment is especially crucial in the U.S., where there simply isn't reliable information on where the disease is and how many people have it.
Another important step would involve moving testing capabilities out of the lab and into doctors’ offices. Ideally, providers should be able to order and run tests rapidly on site as they now can for viruses such as the flu instead of sending them off to an overtaxed lab. An accurate and quick test of this type would mean that fewer people are left in limbo about their infection status, substantially aiding isolation, monitoring, and treatment efforts. People could be diagnosed in a far broader array of settings, lowering the risk of further spread of infection and keeping them out of hospitals that could spend more of their time dedicated to severe cases.
A broader array of diagnostics would enable more targeted surveillance and reduce the need for blanket travel bans and other economically harmful containment measures. FDA support and federal dollars should go to companies and labs working on such tests to get them approved and distributed quickly.
So good news for Roche and the fight against the virus, but we’re still only in the early stages of this outbreak. And we need more firepower.
To contact the editor responsible for this story: Beth Williams at bewilliams@bloomberg.net
This column does not necessarily reflect the opinion of Bloomberg LP and its owners.
Max Nisen is a Bloomberg Opinion columnist covering biotech, pharma and health care. He previously wrote about management and corporate strategy for Quartz and Business Insider.
©2020 Bloomberg L.P.