(Bloomberg Opinion) -- The drug industry is rarely dull, but this week took things to another level.
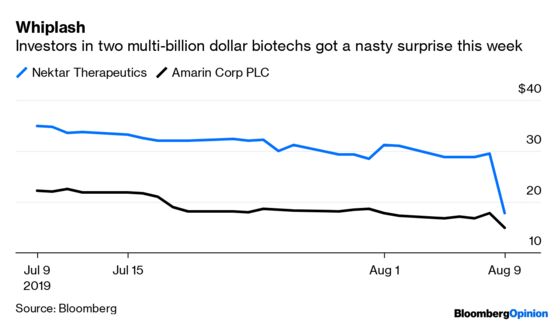
Things kicked off Tuesday with the Food and Drug Administration’s unusual revelation that Novartis AG had delayed disclosing manipulated data related to early animal tests of its $2.1 billion gene therapy Zolgensma. While the drug is still considered safe, the lapse sparked a decline in Novartis shares. A couple of days later, a wild Thursday afternoon created broader biotech turmoil. Amarin Corp.’s stock plunged after it revealed an unexpected delay in the FDA review of new data for its fish oil-derived heart drug Vascepa. Nektar Therapeutics followed by disclosing a manufacturing issue for its key cancer drug that may have impacted trial results. Its shares tanked more than 30% Friday.
Investing in biotechnology can be a tricky business. Stock buyers typically focus on clinical-trial readouts or shifts in drug pricing and health policy, but a whole host of other things can go wrong. Good management and transparency can make things smoother. In the case of this week’s stumbles, spin from company leaders made things worse.
Novartis’s reputation still suffers from an earlier effort to seemingly buy White House access via former Trump fixer Michael Cohen. That should have encouraged the company to err on the side of transparency when it discovered this data issue in March, and let regulators and the public know as soon as possible. Instead, Novartis decided to complete a rather slow internal investigation and didn’t inform the FDA until after the drug was approved two months later.
The data in question came from a test in mice that is no longer in use. Patient safety wasn’t affected, and the drug will rightfully stay on the market. But the FDA relies on drugmakers to provide clean data, especially for drugs like Zolgensma that are on an accelerated path to approval. Breaking that trust is a big deal for a company of this size, and the FDA was understandably livid about the way this was handled. CEO Vas Narasimhan held a conference call and analyst Q&A after the issue became public, but spent much of it defensively downplaying the problem. He didn’t do nearly enough to fix his relationship with the agency or boost investor trust. The problem escalated on Friday after a group of Senators wrote a letter to the FDA blasting Novartis and urging action.
Amarin’s issue wasn’t withholding information from the FDA, but putting words in its mouth. The company said late last month that it believed it was unlikely that the FDA would choose to convene an expert panel to review Vascepa’s ability to cut the risk of heart attacks and strokes. That wasn’t entirely unreasonable as the FDA’s target date for marketing approval on Amarin’s new data was less than two months away. But the FDA sets those dates and can change them. Amarin disclosed Thursday that the agency wants to convene a panel in November after all. That will likely delay approval until at least December. The panel may go swimmingly, and this may be only a minor delay. But investor expectations would have been better calibrated if Amarin hadn’t publicly made risky assumptions.
Nektar’s situation is the strangest of all. On its second-quarter conference call Thursday, management revealed that the company was curious about why some patients weren’t responding as well to its cancer drug bempegaldesleukin. After examining early drug lots used in trials, it found variations in two batches. Nektar said it’s rebuilding its quality-control strategy as a result, which is a good idea; its decision to try to spin a flub that may have harmed patients into an upside case for its medicine wasn’t. The company spent a chunk of its second-quarter call elaborating a confusing post-hoc analysis of the impact of different drug batches. As you might imagine, the way they sliced the data suggests that its drug might have looked more promising if it weren’t for those bad batches. The analysis split already small trials into even smaller new subgroups, which makes the data difficult to interpret. Only a new trial will give investors data they can actually trust. In short, Nektar’s attempt at clarification only muddied the waters.
When confronted with bad news or uncertainty, the desire to spin it is natural. Drugmakers would be wise to resist that urge. When the truth comes out, it just makes the backlash bigger.
To contact the editor responsible for this story: Beth Williams at bewilliams@bloomberg.net
This column does not necessarily reflect the opinion of the editorial board or Bloomberg LP and its owners.
Max Nisen is a Bloomberg Opinion columnist covering biotech, pharma and health care. He previously wrote about management and corporate strategy for Quartz and Business Insider.
©2019 Bloomberg L.P.