(Bloomberg Opinion) -- Covid-19 vaccines may not arrive quite as soon as people hoped. An October surprise didn’t happen, there will be no vaccine by Election Day, and even Thanksgiving might be pushing it.
Front-runner Pfizer Inc.'s oft-repeated suggestion that it might have vaccine data by Halloween was wrong, and Anthony Fauci now suggests an immunization might not be available on even a limited basis until January. Efforts to speed results and availability have been historic, but they have limits. Specifically, quick vaccine hopes have a math problem. To clear a high statistical bar and prove effective enough to get emergency use authorization from the Food and Drug Administration, early candidates would have to be both very protective and quite lucky. That won’t be easy.
Here's how it works. In these Covid-19 clinical trials, some participants get a vaccine, some get a placebo, and researchers measure how many get sick in each group. They examine the data at several points in the trial to see if the vaccinated participants have a lower rate of illness. In order to gain FDA authorization for use against the coronavirus, a vaccine needs to show firm evidence that it protects at least half of those inoculated. Barely clearing 50% isn't good enough to declare success after just a few months, however. This is because at the first checks, when the number of confirmed cases is low, there's a higher statistical likelihood that what looks like a protective effect results from chance rather than the vaccine's good work. The most advanced vaccine trials accounted for this mathematical truth by setting a higher bar for the first look -- efficacy of around 75%.
Just one extra case amid thousands of vaccinated participants could swing early analyses because the numbers are small. That's where luck comes in. Moderna Therapeutics Inc., another leader in the vaccine race, underscored the issue during its third-quarter earnings call last week. The company's presentation noted that success will be essentially a coin flip after 53 cases — when it will take its first peek at the data — even if the vaccine is ultimately capable of preventing Covid-19 in three-quarters of those who get it. If that initial look is inconclusive, the board overseeing the 30,000-person trial won't reassess the shot until cases double. Pfizer faces even more uncertainty because it plans a peek after just 32 confirmed symptomatic infections.
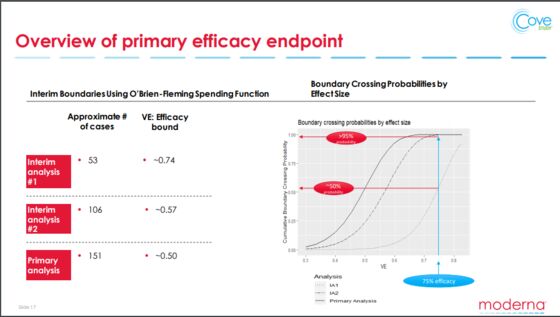
This particular issue doesn’t even come into play until there are enough infections. As of Pfizer’s own third-quarter earnings release last Tuesday, the drug giant hadn't hit its first-look threshold, and its failure to reach just 32 cases in a trial that is larger than Moderna’s suggests participants may be catching Covid-19 less frequently than expected. One possible explanation is "functional unblinding," whereby the presence or lack of side effects might let volunteers guess if they got the vaccine and lead them to take different virus risks, potentially slowing and skewing trial data. Two other leading candidates only just restarted trials after pauses to investigate potential safety issues.
The recent spike in Covid-19 cases may increase the rate of infection in these trials, removing one roadblock in getting to the threshold for looking at initial trial data. But that look will come when the odds of success are at their lowest. What this all means is that the wait for results and FDA authorization may still prove long, and the wait for broad availability and distribution even longer. Vaccines will get here, but anyone thinking it’ll happen anytime soon should re-set expectations.
This column does not necessarily reflect the opinion of the editorial board or Bloomberg LP and its owners.
Max Nisen is a Bloomberg Opinion columnist covering biotech, pharma and health care. He previously wrote about management and corporate strategy for Quartz and Business Insider.
©2020 Bloomberg L.P.