Biogen Has More Hope Than Data for Alzheimer’s Drug
(Bloomberg Opinion) -- No drug for Alzheimer’s disease does anything but treat symptoms of the degenerative ailment. The first medicine that can do more will be an enormous breakthrough.
Biogen Inc. thinks it has that drug in aducanumab. It’s a treatment the company previously announced was a failure in March, but in a highly unusual move, it is trying to resurrect the medicine and gambling that it can win Food and Drug Administration approval. The company presented an expanded case for the medication at a medical meeting on Thursday.
The argument over whether the drug works and is approvable may be the sector’s biggest battleground. Biotech investing is full of so-called binary events — data releases or regulatory decisions that make or break a stock. Most come for startups. Biogen employs 7,800 people, and its market cap has swung by more than $10 billion twice on aducanumab updates. Unfortunately for bulls, Thursday’s presentation did little to suggest that the drug has much of a chance.
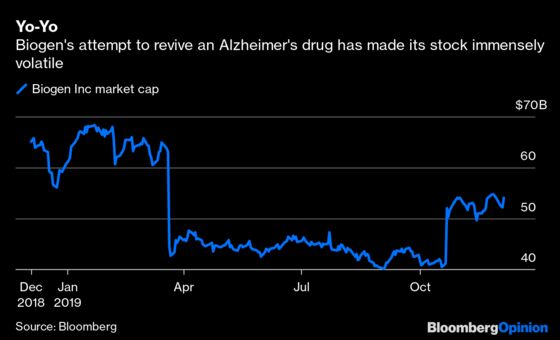
The evidence that Biogen’s medicine works is more confusing than convincing. The company halted two identically designed trials earlier this year after a preplanned analysis found that aducanumab was unlikely to succeed. After looking at new data that came in after the cutoff point for that first analysis, however, Biogen found that aducanumab had a statistically significant impact on cognitive decline in one trial. Even though the second test was still a messy failure, the company decided to push for approval anyway.
This reversal adds all sorts of statistical muddiness to even the positive trial.
If regulators are somehow convinced to ignore those issues, the drug’s impact looks small even in Biogen’s favored set of patients. Add in the failed trial, and the benefit is even more uncertain. The FDA might be more comfortable with that ambiguity if the drug had no side effects. That’s not the case for aducanumab, which can cause brain swelling and microhemorrhages. The burden of proof should be especially high because every other medicine testing the same mechanism for treating Alzheimer’s has failed.
The company further developed its arguments for FDA leniency on Thursday with a significant focus on a theory that a mid-study dosing change and a staggered start time explains the divergence in the two studies. Fancy charts and post-hoc data slicing can’t change the fact that this is a messy trial with inconsistent results. At best, Biogen made a case for running a new clean trial to test its dosing hypothesis, not for putting this drug on the market.
An approval of aducanumab would have a bigger impact than most FDA decisions. It could lead to the spending of billions of dollars in a rapid and widespread uptake of a drug that might not work and could even harm patients. It would also make mincemeat of the FDA’s approval standards and encourage more companies to press forward with questionable data.
Some investors, including those that sent Biogen’s stock up about 4.5% in the aftermath of the presentation, will continue to hope.
The FDA is indeed increasingly flexible when patients don’t have options, and there may be no more significant unmet need than Alzheimer’s. The agency also isn’t immune to political and patient pressure, which will be immense in this case. Biogen’s Chief Scientific Officer Al Sandrock hinted at hard-sell tactics to come, suggesting at a recent conference that the FDA would doom more people to dementia if it required another clinical trial before approval.
The upside from approval would be enormous; the world needs a treatment for Alzheimer’s disease and pent-up demand from millions of patients would most likely generate huge sales. The downside is just as significant, however. If the drug fails, investors will flee a company with flat-lining growth, a weak pipeline and a tarnished management team. Statistics and high standards are likely to win out in the end. Neither are on Biogen’s side.
To contact the editor responsible for this story: Daniel Niemi at dniemi1@bloomberg.net
This column does not necessarily reflect the opinion of the editorial board or Bloomberg LP and its owners.
Max Nisen is a Bloomberg Opinion columnist covering biotech, pharma and health care. He previously wrote about management and corporate strategy for Quartz and Business Insider.
©2019 Bloomberg L.P.