(Bloomberg Opinion) -- After a years-long trial that shocked the pharmaceutical industry, a surprise delay, and regulatory scrutiny, Amarin Corp. finally got the go-ahead late Friday to market its fish-oil-derived heart pill Vascepa to millions of additional Americans.
The expanded approval for prevention of events including heart attacks and strokes isn’t quite as broad as Amarin hoped, but keeps blockbuster sales very much in play. Investors had a muted reaction to the long-awaited news, sending the company’s shares down 2% by midday Monday. The stock’s response looks less worrisome in the context of a 740% increase since September 2018, but it does suggest that much of Amarin’s good news is priced in. Getting in now is a potentially risky bet on a pricey takeover or surprising sales upside.
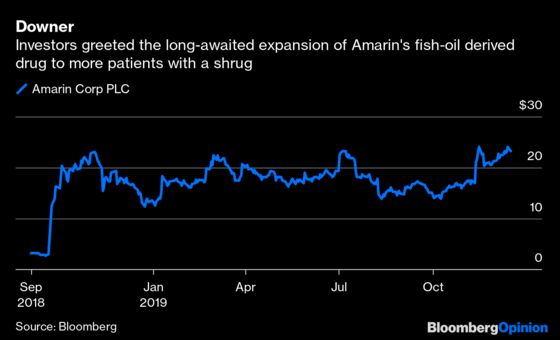
With approval in the bag, Amarin’s next job is selling more Vascepa. The company projects as much as $700 million in sales next year, a big step up from the $400 million seen in a far narrower indication this year. Sales will need to accelerate from there to meet analysts’ high expectations and justify an $8.3 billion valuation. Vascepa certainly has the potential, but it won’t be easy.
The FDA restricted Vascepa to people with high triglycerides who either have established cardiovascular disease or diabetes and other cardiac risk factors, reflecting some experts’ concerns about the drug’s utility in lower-risk patients. That said, it’s still very much a win; Amarin now has access to a huge market. The existence of restrictions will give insurers and pharmacy benefit managers opportunities to slow Vascepa’s growth, however.
Payers may embrace the drug for its relatively low cost and ability to prevent costly cardiac events. Still, the fact that it could reach tens of millions of patients and cause a near-term jump in spending may lead them to throw up barriers to access. Launches of primary-care drugs such as Vascepa are often comparatively slow, marketing-intensive, and expensive. “Sell the launch” is a common refrain from biotech investors. Concern about a shaky start may be behind the negative response to the as-expected details of Amarin’s approval. A few sales beats and guidance boosts may go a long way.
Cash from a deep-pocketed acquirer with commercial expertise would go a long way toward speeding Vascepa’s growth. More investors are likely hoping for a deal than to endure the vicissitudes of a solo heart drug launch. They may have to wait.
Vascepa is a unique and potentially highly lucrative opportunity, there are some risks for a potential acquirer in buying Amarin. It’s hard to imagine the company selling without a solid premium, which could well take the purchase price well past $10 billion. Drugmakers typically don’t cross that threshold for a biotech target lightly.
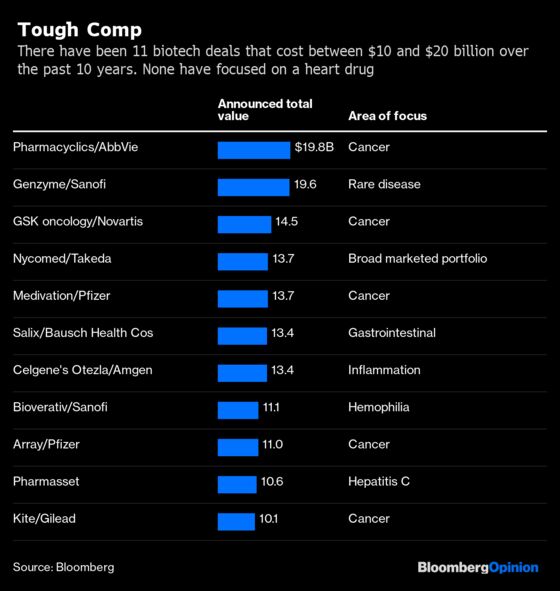
Big Pharma has been increasingly gravitating toward higher-priced and easier-to-launch rare-disease and cancer drugs, and away from medicines like Vascepa. While Novartis AG at least has gone the other way with its recent $9.7 billion acquisition of Medicines Co. for long-acting cholesterol medicine, other potential acquirers may be leaning closer to Sanofi, which just gave up entirely on researching cardiovascular and diabetes drugs. Possible patent and competitive risks may be creating some hesitation as well.
Amarin’s approval is still historic and highly promising. The market just needs a bit more proof that it will actually translate into revenue and returns.
To contact the editor responsible for this story: Beth Williams at bewilliams@bloomberg.net
This column does not necessarily reflect the opinion of the editorial board or Bloomberg LP and its owners.
Max Nisen is a Bloomberg Opinion columnist covering biotech, pharma and health care. He previously wrote about management and corporate strategy for Quartz and Business Insider.
©2019 Bloomberg L.P.