Astra Covid-Shot Data Leaves Some Analysts Questioning It
Results from a crucial study of AstraZeneca Plc and the University of Oxford’s Covid-19 vaccine drew a harsh review.

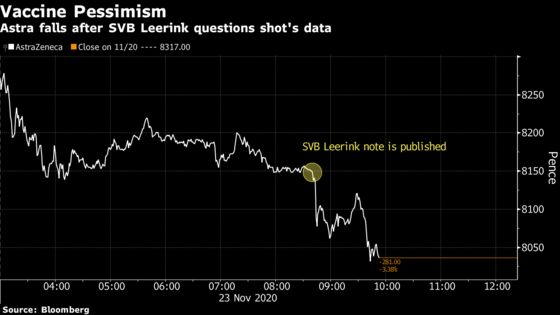
(Bloomberg) -- Results from a crucial study of AstraZeneca Plc and the University of Oxford’s Covid-19 vaccine drew a harsh review from at least one sell-side analyst as Wall Street grappled with the future of a potentially less effective shot.
The results showed the vaccine stopped an average of 70% of patients from falling sick. However, the company’s formatting of the data that highlighted a 90% effectiveness drew skepticism from SVB Leerink analyst Geoffrey Porges. The analyst said the company highlighted results from a “relatively small” group of volunteers in the trial and wouldn’t get U.S. approval based on a lack of diversity among participants, he wrote in a note.

Ruud Dobber, head of Astra’s biopharmaceuticals business unit, said SVB Leerink’s comments were harsh. “I think it’s far too early to speculate about how regulators will react,” he told Bloomberg TV in response to questions on the note.
SVB Leerink holds an outperform rating on Astra with a $65 price target.
Still, Astra shares fell as much as 3.3% in London trading to the lowest level in almost three weeks, while the company’s U.S-traded shares slid 3.2% to $53.47 in early trading. Despite the mixed reaction from analysts, U.S. stocks edged higher amid signs of progress toward a Covid-19 vaccine with the S&P 500 up 0.1%.
Porges also said Astra and Oxford officials would be “roundly criticized” for a safety disclosure that was “hardly reassuring.” The U.S. arm of the study was paused for almost seven weeks in September after an adverse event involving a participant in the U.K.
U.S. trials in the study are working on a two-dose regimen currently and about 10,500 participants have already been given both shots. Astra and Oxford said they will talk to the U.S. Food and Drug Administration this week about setting up a separate arm of the trial to test the more effective, lower dose regimen, and conceded this could affect the speed of approval in the region.
Porges wasn’t alone on Wall Street in questioning the results from Astra and Oxford. Jefferies analyst Michael Yee called the data “mixed” and highlighted that results from competitors including Pfizer Inc. and partner BioNTech SE as well as Moderna Inc. looked more robust.
While the Astra and Oxford program had positive effectiveness, Yee questioned which countries would prefer to use the vaccine that has notably lower efficacy. “Having any cases of Covid implies big risk, so why not use the best (vaccines), and which populations of citizens would be OK knowing they are getting one that has notably lower efficacy?” Yee asked.
Storage Advantage
Officials from Oxford and Astra said at a press briefing this morning they didn’t yet know why the lower dose produced a better response and would be looking to understand this better as they dig further into the data, which will be peer-reviewed over the coming weeks.
Despite not matching the success of frontrunners Pfizer and Moderna, one thing working in Astra’s favor is storage. The Astra-Oxford shot only needs to be kept at fridge temperature, unlike the other two which must be kept frozen for long-term use. That makes Astra’s vaccine much more important for the developing world.
Some drug developers that are racing to bring a shot to the market that can help end the pandemic rallied after Astra and Oxford’s results. BioNTech and Moderna each gained 2.8% while Novavax Inc. climbed 2.7%.
©2020 Bloomberg L.P.