U.S. Buys Almost All Abbott’s $5 Rapid Tests Made This Year
A 15-minute Covid test from Abbott that will be priced at just $5 has been granted emergency authorisation for use in the U.S.

(Bloomberg) -- The U.S. government is buying almost all of the $5 Covid tests Abbott Laboratories plans to produce this year, purchasing 150 million of the tests that were cleared yesterday and can be run in just 15 minutes.
Under the agreement, the government will pay $750 million for 150 million tests, according to people familiar with the deal. The company was granted an emergency authorization on Wednesday, and public health officials quickly agreed the new test -- which works without relying on laboratory equipment -- could help ease delays that have crimped much of the nation’s testing capacity.
The Trump administration has been criticized for failing to institute a coordinated testing approach in the U.S., and for a recent change in guidelines that curbs the testing of people without symptoms. Now, it plans to purchase almost all of the coming supply of a breakthrough product that can be used broadly, and promises to deliver results on a mass scale no matter where it’s given.
Unlike other tests, Abbott’s BinaxNOW is entirely self-contained and doesn’t need other equipment to get results, meaning large numbers of tests can be done simultaneously. It’s a single-use test about the size of a credit card, with results given at the point of care.
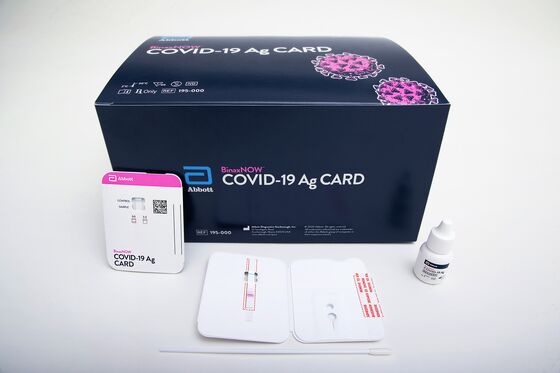
Abbott has said it will produce 50 million a month starting in October and plans to sell them widely for $5 each. It expects to have “tens of millions” for September, said Andrea Wainer, executive vice president of the Rapid & Molecular Diagnostics unit. Abbott spent hundreds of millions of dollars on the technology, infrastructure and manufacturing for BinaxNOW and its other tests, self-funding the innovation, the company said in a statement.
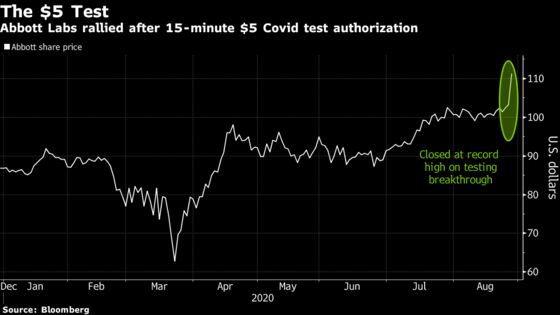
Shares climbed 7.9% Thursday to $111.29 at the close of New York trading.
“This is a major development that will help our country to remain open, get Americans back to work, and kids back to school,” said Alyssa Farah, the White House communications director. “The Trump administration is proud to partner with Abbott labs to make this purchase possible to help the American people.”
It’s not entirely clear who will receive the initial round of tests. An administration official said they will go first to nursing homes, schools and other high-risk populations, but details were scant. Demand is expected to be high, as they could help guarantee the safety of everything from flights to restaurants to offices.
Public health officials at the state level are already trying to get access.
Jared Moskowitz, director of Florida’s division of emergency management, said on Twitter he is “aggressively” pursuing the test, which he called a game changer.
The test uses so-called lateral flow technology, similar to the method used in at-home pregnancy tests. It can be administered by a range of health-care workers, including pharmacists or even school nurses, in almost any location.
The provider swirls a collection swab through both nostrils, then inserts it into the BinaxNOW card and adds a few drops of a liquid chemical known as an extraction buffer. The card is closed and the liquid sample flows along the surface of a pad that has reactive molecules embedded, which gives a result in 15 minutes.
While a pregnancy test detects a hormone, BinaxNOW looks for an antigen, a tiny portion of the coronavirus protein.
Antigen Controversy
It’s not the first round of controversy over fast-acting antigen tests. Several other companies, including Quidel Corp. and Becton Dickinson & Co., also have received authorization for similar diagnostics, though they require small pieces of equipment to analyze the results.
The federal government is also buying large numbers of those devices to send to nursing homes as part of its efforts to get the virus under control in high-risk areas.
The result has been conflict between the U.S. government and other groups seeking to enhance their testing.
Tommy Thompson, the interim president of the University of Wisconsin System and former Secretary of Health and Human Services under George W. Bush, said he had to fight to get 36 of the Quidel machines that it ordered to help it open campuses across the state after the federal government stepped in.
With the latest tests, Thompson said he understood why the administration was cutting to the front of the line.
“Everybody is looking for a faster, cheaper, better test,” he said in an interview. “The federal government is going to take a big chunk of it, and they should. But we want as many as we possibly can get,” he said. “We are already in line with Abbott, and hopefully we’ll get some of this test as well.”
Soaring Demand
Industry watchers expect soaring demand for the technology that could help Americans get out of their houses more often and more safely.
Demand for an antigen test “could be ENORMOUS -– think about employers, restaurants, games/events, transportation etc. using these tests for ensuring safety,” Vijay Kumar, an analyst at Evercore ISI, wrote in a note to clients.
The test could help people return to a sense of normalcy while helping generate $2.7 billion to $2.8 billion a year in revenue for all Covid-19 products at Abbott, said Robbie Marcus, an analyst at JPMorgan, in a note.
Abbott Chief Executive Officer Robert Ford acknowledged the expected demand in an op-ed published today by CNBC. The product is transformative because it can be made in large numbers, “which is what the country needs more than anything today,” he said.
Abbott is also launching a mobile app called Navica that will be connected to the test, giving users an electronic record of their coronavirus status. The results could be used much like a boarding pass to allow those who are negative to return to everyday activities.
Required to Report
Those with a positive result will be told to quarantine and contact their doctor. Health-care workers who conduct the tests are required to report positive results to public health officials.
The U.S. is currently running about 800,000 tests a day nationwide, or 24 million a month, according to the Covid Tracking Project. Abbott built two new manufacturing facilities in the U.S. to produce BinaxNOW, allowing it to more than double the number of tests available to 50 million a month.
“Our nation’s frontline health-care workers and clinical laboratory personnel have been under siege since the onset of this pandemic,” said Charles Chiu, a professor of Laboratory Medicine at University of California, San Francisco. “The availability of rapid testing for Covid-19 will help support overburdened laboratories, accelerate turnaround times and greatly expand access to people who need it.”
Who exactly those people are has yet to be determined.
©2020 Bloomberg L.P.