Trump’s FDA Delayed Regulating Vaping Until the Crackdown
Trump’s FDA Delayed Regulating Vaping Until the Crackdown
(Bloomberg Businessweek) -- Something was buried in the press release the U.S. Food and Drug Administration put out on July 27, 2017. The agency was making an historic announcement: the initiation of “a multi-year roadmap to better protect kids and significantly reduce tobacco-related disease and death.” It quoted FDA then-Commissioner Scott Gottlieb: “Unless we change course, 5.6 million young people today will die prematurely later in life from tobacco use.” While focusing on combustible cigarettes that deliver nicotine via smoke particles, the FDA recognized that e-cigarettes needed regulation as well. In the seventh paragraph, it announced coming “revised timelines” that would require e-cigarette makers to apply for approval by Aug. 8, 2022—a long extension from the then-existing FDA deadline of August 2018. “The FDA expects that manufacturers would continue to market products while the agency reviews product applications,” it said.
And that’s what the industry did. In the past two years, the market for vaping—which uses flavored vapor, not smoke, to deliver nicotine—has almost doubled, to an estimated $8.8 billion, in 2019. Leader Juul Labs Inc., which last year got a $13 billion investment from Marlboro maker Altria Group Inc., accounted for nearly 1 in 3 e-cigarettes sold in the U.S. as of December 2017. The company’s sales increased 641% over that year, to 16.2 million devices, according to data from the Centers for Disease Control and Prevention.
Contributing to the surge are two things: the belief that vaping is a safe and effective way for adult smokers to quit their cigarette habits, and the appeal to young people of candylike flavorings and sleek technology available in the electronic devices. Sales grew even as the regulatory schedule meant there would be a huge gap in the public’s knowledge about the safety of e-cigarettes.
For months, members of Congress had petitioned for speedier regulatory action by the FDA. Gottlieb drew up plans, though few have been implemented; he stepped down in April and is now on the board of the pharmaceutical giant Pfizer Inc., which makes the smoking cessation drug Chantix. Then, over the summer, a mysterious lung disease linked to vaping killed six people, and U.S. health officials are now investigating more than 380 confirmed and probable cases. In July a judge moved up the regulatory deadline for e-cigs to May 2020. But as the negative news surrounding vaping grew, concern within the administration mounted.
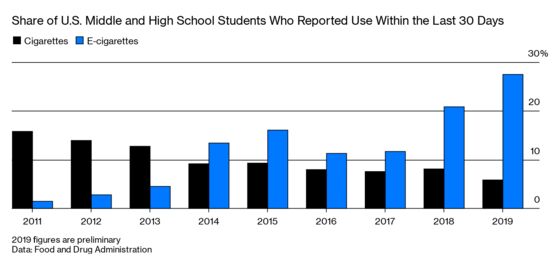
On Sept. 11, President Donald Trump and Health and Human Services Secretary Alex Azar said the FDA will move to take all nontobacco-flavored e-cigarettes off the market and make manufacturers await regulatory review to determine whether vapes are good for public health. On the same day, the FDA released preliminary 2019 data from an annual youth tobacco survey that found about 5 million under-18 users vaped within a 30-day period. That’s up from 3.6 million in 2018. Gottlieb hasn’t yet been officially replaced at the FDA, which is led by acting Commissioner Ned Sharpless, previously the head of the National Cancer Institute.
Back in 2017, Gottlieb said he delayed e-cigarette reviews because the agency wanted “to make certain that the FDA is striking an appropriate balance between regulation and encouraging development of innovative tobacco products that may be less dangerous than cigarettes.” The regulatory lapse, Trump said in reversing the policy, “frankly, should have been looked into a few years ago in a much more advanced way.” Azar attempted to pin that time frame on the Obama administration, saying it “allowed these products to go onto the market in an unregulated way.” Congress had given the Obama administration the power to regulate e-cigarettes in 2009. But, while it was slow to do so, it was Trump’s FDA that pushed back the deadline.
Just before stepping down, Gottlieb told the audience at a forum hosted by the Brookings Institution that he wouldn’t have done the same thing if he knew what he knows now about the vaping epidemic. “Absolutely not,” he said. He keeps coming back to the point: “Had we known what we know now, or learned in 2018,” he says today, “there’s little doubt we would have made different decisions.” He adds that one of his reasons for pushing back the review was that the FDA hadn’t yet created application guidelines, which are only now being finalized.
Still, in September 2018, reports of a marked increase in youth vaping led Gottlieb to threaten a stop to sales if five major e-cigarette manufacturers didn’t come up with plans to stem what he called an “epidemic.” He met with representatives of Juul and Altria. He followed up in March 2019—not by stopping sales, but by proposing to move the deadline for review of flavored e-cigarettes up a year to August 2021. After Trump and Azar announced plans to dramatically revise the administration’s e-cig policy, Gottlieb tweeted, “If I seem personally upset by this turn of events today, it’s because I’ve watched Juul actively work to try and thwart public health efforts to get better regulation over products that we know are hurting children.” Juul said in an emailed statement that it supports “aggressive category-wide action on flavored products” and would comply with any forthcoming FDA guidance.
It’s unclear exactly when this will arrive. Azar said the FDA is in charge of issuing guidelines, which he expected would take several weeks. But details were sparse, and nothing is yet on paper. Indeed, who’s in charge of the new policy and its formulation is unclear. By law, Sharpless’s term as acting commissioner is up on Nov. 1, and Trump may choose not to nominate him to head the agency permanently. The president has been considering Stephen Hahn, who helps run the MD Anderson Cancer Center, for the job. And once Trump makes his choice, it will take time for the Senate to hold a hearing and vote to confirm the nominee. Many legislators are distracted by the long runup to next year’s presidential elections.
Scientific investigation into the risks continues. Health officials have warned that vapes that include THC, the active ingredient in marijuana, have largely been to blame for the illnesses, but they haven’t ruled out nicotine vapes. Meanwhile, the vaping industry has time to try to water down any restrictions. Last year, Josh Raffel, a former White House spokesman who was close to Jared Kushner and Ivanka Trump, joined Juul as spokesman. The company also hired Johnny DeStefano, a former Trump adviser, as an outside consultant.
In recent months, Juul has said it’s trying to keep kids away from addictive flavors such as mango and cucumber and blamed counterfeit flavor pods that work with its devices for spurring underage vaping. That was echoed by the White House just two days after Trump’s tough talk. The president tweeted: “While I like the Vaping alternative to Cigarettes, we need to make sure this alternative is SAFE for ALL! Let’s get counterfeits off the market, and keep young children from Vaping!”
To contact the editor responsible for this story: Howard Chua-Eoan at hchuaeoan@bloomberg.net
©2019 Bloomberg L.P.