One-Shot Drug to End Sicilian Curse Comes at $1.8 Million Cost
One-Shot Drug for Sicily’s Rare Blood Disease Costs $2 Million
(Bloomberg Businessweek) -- On a sweltering summer morning, Daniela Miccichè headed from her home in the center of Sicily to a hospital in the ancient city of Palermo on the coast. It’s a trip she repeats every month. A travel agent from the town of Caltanissetta, Miccichè has no choice but to make the two-hour journey to the Franco and Piera Cutino clinic. Scores of others pass through its front doors, all with the same disease. She’s among an estimated 7,000 Italians, many of them Sicilian, who depend on transfusions to treat beta thalassemia, a genetic disorder that hampers red blood cells’ ability to carry oxygen.
Miccichè has waited a long time for the day she can be free from these procedures, a constant in her life since she was 3 years old. “I grew up thinking that one day we would reach this goal,” she says.
Patients such as Miccichè are close to having their wish granted, but it’s going to cost the Italian medical system a huge sum. A novel gene therapy from U.S.-based Bluebird Bio Inc., approved in Europe in June, is set to be rolled out next year following reimbursement negotiations with government health systems. The treatment, called Zynteglo, has a sophisticated mechanism and a lofty price tag—€1.58 million ($1.8 million) per person—which puts into sharp focus an increasingly common dilemma.
Dozens of gene therapies for a range of devastating illnesses are on their way. These single-dose drugs, tailored to each patient, can potentially deliver a lifetime of benefits. But that’s reflected in their prices, which are likely to increase pressure on already stretched budgets. To make it easier for government payers to digest Zynteglo, Bluebird plans to spread out the cost over five years, with payments contingent on its success.
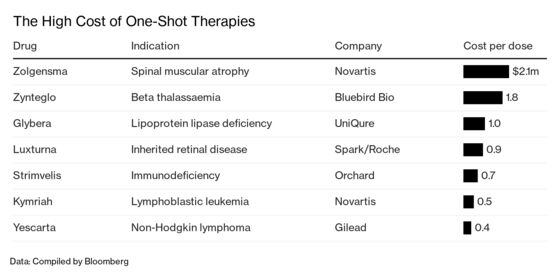
As a one-time therapy, Zynteglo could save governments money in the long run by cutting the need for expensive ongoing care. Treating one beta thalassemia patient today can cost as much as €60,000 a year, says Aurelio Maggio, a blood-disease specialist at the Palermo center. That’s €3 million over five decades. With multiple wonder drugs for other conditions set to reach the market soon, the upfront bill could take a heavy toll on Italy’s finances. The price tag for the therapy is twice the $900,000 that SVB Leerink analyst Mani Foroohar expected. Given the large number of patients in the country with the ailment, “the stakes are much higher,” he says.
Regulators predict there could be as many as 20 cell and gene therapy approvals annually by 2025. Novartis AG’s Zolgensma, which was approved in the U.S. in May for a childhood illness called spinal muscular atrophy, is priced at $2.1 million. U.K.-based Orchard Therapeutics Plc is also developing a treatment for beta thalassemia, which afflicts almost 300,000 people globally. Zynteglo could gain approval in the U.S. next year and reach $1 billion in annual sales in 2025. “We’re benefiting from an explosion of innovation and have crossed a lot of the key scientific hurdles,” Foroohar says. “Getting paid for that requires slower-moving entities such as governments, payers, and regulators to catch up.”
In Italy, adoption of Zynteglo could have a significant impact. More than twice as many of its citizens suffer from beta thalassemia as in France, Germany, the U.K., and the U.S. combined, Bluebird estimates, and many live on the islands of Sicily and Sardinia. But fewer than a third of Italian patients may be immediately eligible, because regulators approved it only for those 12 years and older who don’t have a serious type of the disease that leaves them unable to make any hemoglobin, the key protein in the blood. Paying for a limited number of people initially will make it easier for governments to afford, says Andrew Obenshain, head of Bluebird’s Europe business. The company intends to eventually reach a broader pool of patients.
People with beta thalassemia, which can lead to life-threatening anemia, extreme fatigue, and other problems, require transfusions as often as every two weeks. One side effect is excess iron, resulting in possible liver and heart damage if untreated. The standard therapy has improved over the years, but the prospect of a cure “represents a dream for the thalassemia population,” says Franco Locatelli, a doctor at Sapienza University of Rome who was involved in the Zynteglo trials.
The blood disorder, related to sickle cell disease, has burdened Sicily for centuries and is common in Africa, Asia, and other regions where malaria historically was also prevalent. Scientists say the gene thrived because it offered protection against the mosquito-borne illness. Carriers who inherited only one copy of the flaw were more likely to survive and pass on the gene to their children, leading to higher prevalence in successive generations.
Bluebird, based in Cambridge, Mass., spent decades developing a way to use a modified virus to transport a functioning version of the gene into a patient’s cells to restore production of hemoglobin. That work has finally paid off. After refining the process for manufacturing the therapy, the company is working with governments to hammer out coverage based on its novel five-year installment plan. The drugmaker expects to introduce Zynteglo in Europe in 2020. Getting the product to patients in Italy will likely take about a year from the time it was cleared, Bluebird’s Obenshain says.
In the meantime, patients and family members are eager to get the drug. The therapy gives Valentino Orlandi new hope as his 60th birthday looms. The Ferrara-based entrepreneur may be able to spend the rest of his life without the burden of blood transfusions. “We have been waiting for Zynteglo for over 40 years,” he says. “We are now in a hurry.” —With Luca Casiraghi and Albertina Torsoli
Read more: How Parents Get Zolgensma, the World’s Most Expensive Drug
To contact the editor responsible for this story: James Ellis at jellis27@bloomberg.net, Rick Schine
©2019 Bloomberg L.P.