Making New Elements Doesn’t Pay. Just Ask This Berkeley Scientist
Making New Elements Doesn’t Pay. Just Ask This Berkeley Scientist
(Bloomberg Businessweek) -- So here we are, at the edge of chemistry: atomic No. 118, oganesson, the coda of the periodic table as it stands and the spot where questions of science shade into those of philosophy. Is an element really an element if it exists nowhere in nature—if it can be engineered only in a lab for a fraction of a second before it blinks out of existence? Is “discovery” the right word for such a feat? How far can scientists extend the table by mashing lighter elements into each other to create heavier ones? Is it even worth the time and trouble to do it just to add a square?
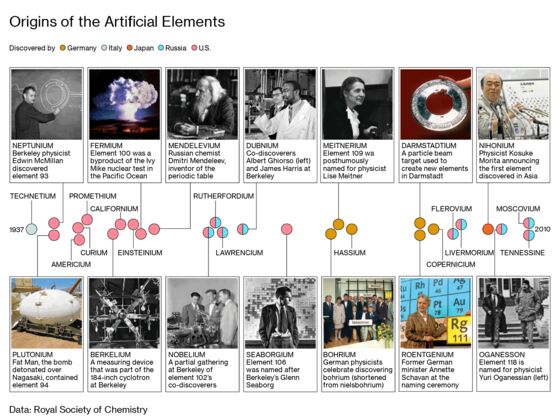
One place to find answers to these questions is on Cyclotron Road, a calf-busting walk up a hill above the University of California at Berkeley campus. For decades, Berkeley’s tremendous accelerators—which hosted within their guts the collisions and combinations of high-energy particles—produced a parade of elements the world had never known: 15 of them, including every element save one from atomic No. 93 through 106. They came to be called superheavies, though this was always an imprecise term, referring variously to elements above 92, or above 100, or above 103.

Even in their nomenclature, Berkeley cornered the glory. Two superheavies were called lawrencium and seaborgium, named after Ernest Lawrence and Glenn Seaborg, Berkeley’s titans of nuclear physics, who’d built the lab’s accelerators and deployed them to study the heaviest elements of the periodic table. Two others were called californium and berkelium, prompting a New Yorker wisecrack that, by not naming another pair “universitium” and “ofium,” Berkeley had squandered its chance at true immortality.
But then the parade stopped. The last new element synthesized at Berkeley was seaborgium in 1974. Announcements of fresh superheavy discoveries were now made by labs in Dubna, Russia; Darmstadt, Germany; and Saitama, Japan. The science changed, and it seemed as if Berkeley wasn’t even trying to catch up. The expense of making elements climbed into the millions of dollars—money that could never be recouped, because most superheavies don’t remain stable long enough to be commercially viable. Funding withered away; once, while having to build an ion separator out of spare parts, Berkeley’s scientists fashioned a valve out of a mousetrap spring. Some of the lab’s accelerators were decommissioned. One was replaced by a parking lot.
In the late 1990s a Berkeley team pushed one final time beyond the boundary of the periodic table, even declared that it had stumbled upon 118—only for the data to be exposed as one man’s scientific fraud. And that was that. Its scientists joined others pursuing new elements elsewhere, but in their own particle accelerators, they applied themselves only to investigating the known superheavies. Among the half-dozen or so major institutions engaged in this work, Berkeley alone has decided to stop chasing the tail of the table altogether.
Nothing in science is the same blend of mundane arithmetic and mystical alchemy as the manufacture of new elements. The protons in an atom’s nucleus determine its atomic number and thus its identity as an element. Melding the atoms of different elements will produce a heavier element, its atomic number predictably just the sum of those of the lighter ingredients. To synthesize seaborgium (106 protons), you might fuse chromium (24 protons) with lead (82 protons) or oxygen (8 protons) with californium (98 protons). A first grader could do those sums. She might not know, though, that uniting two atoms of iodine (53 protons) or other elements of similar size requires unachievable power, or that it’s best to use a lighter atom as a missile trained upon a heavier target. To determine which reaction has the healthiest chance of success, and to calculate the speeds at which the atoms must collide, and to then rev up a particle accelerator and establish those conditions of screaming energy and infinitesimal precision, and to thus forge an element that’s never appeared in the history of the universe, or has perhaps only briefly materialized in the core of a distant star—that feels like an act of cosmic creation.
There’s still a particle accelerator at Berkeley that dates to the heyday of its search for new elements: a cyclotron with a chamber 88 inches in diameter, built in 1961 and set deep within a building on 1 Cyclotron Rd. Berkeley only manages the cyclotron. It’s always been the property of the U.S. government—first of the Atomic Energy Commission and then of its successor, the Department of Energy. Anyone who wants to meet Jacklyn Gates must make an appointment, present an ID to the guards manning the boom gate across the road, walk to the door of the building, call her on a house phone, and wait for her to come down and open the door.
As a staff scientist in the heavy-element chemistry program, Gates uses the cyclotron as her chief instrument, designing tests and running them for weeks. I visited her in May, the day before the cyclotron was going to be booted up for a week and a half of experiments on mendelevium, element 101. A helium ion, with its two protons and two neutrons, would be fired at a nibble of einsteinium (element 99) smeared on metal foil, and the two would then combine into any of the 16 isotopes of mendelevium. The isotopes all hold 101 protons, but their atomic masses differ because they have varying numbers of neutrons. “We’re looking at a couple of different isotopes to see whether or not their masses have been properly assigned,” Gates said. “And we hope to learn something about how the nucleus looks, what shape it is, or how the neutrons and protons are arranged.”
She led me through 10-foot-thick concrete walls into the steampunk heart of the cyclotron: wires and tubes and display lights everywhere, pipes leading from chamber to chamber, giant magnets to accelerate the ions to a third of the speed of light and then to bend and guide them toward their targets. The air was filled with a constant, almighty whirring. It sounded as if the cyclotron was indulging in a last, long yawn before shaking itself awake to work.

Gates came to Berkeley for a Ph.D. in 2004, so she missed the fraud scandal that exploded in the late ’90s. Victor Ninov, a Bulgarian scientist who’d helped find elements 110, 111, and 112 at the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt, had been hired away by Berkeley in 1996 to be a shining talent in the team’s renewed quest for new elements. The lab had acquired a sophisticated separator, an apparatus to pick out the stray superheavy atom formed amid the trillions of particles tearing around in an experiment. A breakthrough seemed eminently possible. Ninov coded software to analyze the data spilling out of the cyclotron, and in 1999, after a project of bombarding a lead target with krypton, his program revealed the formation of element 118.
Berkeley made the announcement in jubilation; it signaled a return to the fruitful years of Lawrence and Seaborg. The International Union of Pure & Applied Chemistry (IUPAC), which vets such findings, waited for further confirmations, but other labs failed to replicate the experiment. When Berkeley’s scientists picked through the raw data, they learned it had been rigged to produce results. Ninov maintained that he never falsified anything, but he was nonetheless fired in 2002. In the years thereafter, scientists elsewhere discovered elements 113 through 118, and Gates traveled to Darmstadt to collaborate in some element-hunting expeditions. But Berkeley launched no searches of its own. “We kind of shied away from that,” she said. “It could have been part of the fallout from the 118. I think we just weren’t interested in taking that up again.”

Gates’s mendelevium experiment is typical of the kind of research into known superheavies that Berkeley now conducts. “Pretty much the only thing we know about these elements is how to produce them, and how long they live, and how they decay,” she said. “What we don’t know is everything else.” She fits her projects into the windows in the schedule left by the cyclotron’s other main function: auditioning microchips to see how well they withstand radiation before they’re installed on satellites. The chip testing keeps the facility funded; in May, Boeing, Blue Origin, and NASA had all paid for chunks of cyclotron time. That was the mandate from the Department of Energy, according to Gates. Even if Berkeley’s scientists wished to pursue new elements, it would be difficult to commandeer the particle accelerator for long enough to run their experiments.
Enlarging the periodic table requires time and money, and scientists have to be prepared to see both resources wasted. In his new book, Superheavy: Making and Breaking the Periodic Table, Kit Chapman, a British science journalist, recounts how the Berkeley team spent a month trying to confirm GSI’s discovery of 112, running the cyclotron for $50,000 a day, only to find that their magnets weren’t properly tuned. In the mid-2000s, Vanderbilt University physicist Joseph Hamilton was working with a Russian team to synthesize 117, traveling often to Dubna. They needed a berkelium target, so he turned to Oak Ridge National Laboratory in Tennessee, which manufactures superheavies for commercial and research purposes—actinium for cancer drugs, californium for the oil industry, plutonium to power space missions. How much would it cost to provide enough berkelium for the experiment? The estimate: $3.5 million.
Hamilton waited instead for Oak Ridge to receive a large order for californium so he could buy the berkelium that would emerge as a side product. “Every three months, for three and a half years, I called them to ask if they’d had any orders,” Hamilton says. The eventual price tag was still about $600,000. He wrote a research grant for most of the cost, then offered a Berkeley lab a buy-in to the experiment for the remaining $100,000.
Hiromitsu Haba, a nuclear chemist at Riken, a research institute in Japan, says that to create just three atoms of element 112 over nine years cost $3 million in electricity bills, supplies, and salaries for the technicians who ran the accelerator. Riken is government-funded, but Haba’s team defrayed the expense by pitching the prestige of the project to the companies that supplied them with hardware, giving them the chance to be involved in an historic mission. (The companies offered discounts or sponsored slices of the budget.) Until Riken ran down its quarry and named it nihonium, Haba explains, “out of the 100 or so elements that existed, not one had been discovered in Asia.”
Like heavy-element labs everywhere, Haba’s team had to share accelerator time with companies manufacturing radioactive isotopes for medical use or researchers studying different elements. In all, across nine years, just 200 days were allotted to the nihonium experiments. Panning for new superheavies is an exercise in probability and patience. “If you’re very lucky,” Haba says, the desired reaction will “happen in the first day.”

Matthias Schädel, who was a nuclear chemist at GSI for almost four decades until 2010, remembers the frustrations well. First, he had to persuade colleagues to collaborate, and then he had to apply for funding and for time with the accelerator. “Sometimes this preparatory phase takes several years,” he says. When a beam of projectile ions finally begins to shoot toward its target, the scientists’ work enters a monotonous routine: checking settings, monitoring the beam. “Especially during night shifts, this can be a dull phase,” Schädel admits. His colleagues read journals or novels in between casting an eye over the data cascading down a computer screen. Once an hour or so, they checked the beam to make sure it was still firing true. It got tiring, particularly by the time “the nth night shift in a several-weekslong experiment” rolled around. What jolted you wide awake, he says, was if the data suddenly revealed an “event”—an unusual nuclear reaction that might, after weeks and weeks of further analysis, turn out to be the signature of an unfamiliar element. Schädel pulled plenty of these night shifts, but he was never part of the discovery of a new element.
Given these steep investments of time and funds, Gates wonders if the search is always worth it. “My personal opinion is that in the amount of beam time it takes to make a new element, you could learn a ton about the superheavies that we’ve already made.” Of course, she said, students in school don’t learn who gauged the ionization potential of lawrencium; they learn who first made the element. “So if you want to make a bigger impact in public, you would make a new element, because that’s a much flashier experiment. The politics starts driving it instead of the search for science.”
The periodic table was never expected to furl out endlessly. In these extreme reaches of the table, cramming proton after proton into a nucleus renders it more and more precarious. The positive charges repel one another until the nucleus decays near-instantly—before electrons have had a chance to settle into orbit to provide an atomic structure and before the passage of a hundred-trillionth of a second, the time an atom must exist to count as a new element. Were you to reach element 173, scientists theorize, matters could get even stickier. The effects of Einsteinian relativity would kick in, and electrons would behave in peculiar ways. Those atoms may not even be atoms as we know them—their electron clouds dissolving and the regular periodicity of their properties swerving wildly off course.

But physics presents difficulties long before 173. Even for 119, waiting just offstage, scientists aren’t sure which two elements they might fuse. Oganesson, No. 118, was the product of an especially stable isotope of calcium slamming into californium. But that calcium can’t just be directed toward einsteinium, the next element after californium; a handful of nuclear reactors around the world generate only a milligram or so of einsteinium for research every year. Seven years ago at GSI, Christoph Düllmann and his team tried a combination of titanium (22 protons) and berkelium (97 protons), without results. In Japan, Haba has been working with vanadium (23 protons) and curium (96 protons). In a $60 million Superheavy Element Factory in Dubna, inaugurated in March, scientists are pelting berkelium with an extra-stable titanium isotope, its nucleus fat with six neutrons more than standard titanium. But at the moment, Düllmann says, 118 “is the end of the story. We now need one more idea. Maybe we’ll get enough einsteinium at some point. But we have no idea what combination of elements is best for 119 and 120. The number of theories is the same as the number of theorists you talk to.”
The theorists agree that 119 and 120 are probably within reach. Elements tend to be discovered in bunches, says Paul Karol, a nuclear chemist who chaired IUPAC’s working group on new elements. “Right now there’s a gap, but it hasn’t died out. It’s just pausing for breath.” Beyond 120, everything is contested. Some scientists speak hopefully of coming upon an “island of stability,” a group of elements holding such ideal numbers of protons and neutrons that they’re magically stable, deigning to stick around for hours or days or even years. “But it’s sure going to be tough,” Karol says. “You’re trying to head in a certain direction, and there’s a strong wind blowing you off course. It’s possible that you won’t make anything—that you drown in the sea and not land on the island.”
And at that point, at long last, the campaign to expand the periodic table will come to a halt. It won’t be for reasons of material utility; the synthetic elements stopped being of any practical use around No. 98. Rather, their value always lay in the research they engendered: the design of experiments, the careful consideration of beam speeds, and the study of the physical properties of these fugitive atoms. “You’re training people who can step aside from everyday science and come up with something that’s new,” Karol says. “You’re feeding minds with this stuff.” But if elements stop revealing themselves, research on the frontier dries up as well. Grants will find new ventures, and scientists will follow them; cyclotrons will be turned into other facilities, or perhaps into more parking lots. For the first time since 1869, when Dmitri Mendeleev stood before the Russian Chemical Society and proposed a novel way to arrange elements, his periodic table will cease to be an unfinished work, a map with borders yet to be filled in.
This story is from Bloomberg Businessweek’s special issue The Elements.
To contact the editor responsible for this story: Bret Begun at bbegun@bloomberg.net
©2019 Bloomberg L.P.