AstraZeneca Hopes New Data Gets Its Covid Vaccine Back on Track
AstraZeneca Hopes New Data Gets Its Covid Vaccine Back on Track

(Bloomberg Businessweek) -- Tens of millions of people around the world are desperately trying to get their hands on a potentially lifesaving coronavirus vaccine. But a group of irate private-sector doctors in Italy is appealing to the country’s health ministry to avoid having to take the Covid-19 shot it’s offered them: the AstraZeneca Plc inoculation, which they believe is less effective.
Their objection speaks to the growing backlash in Europe against the vaccine co-developed by Astra and the University of Oxford. Professionals working in Italy’s public-sector health system received vaccines from Pfizer Inc. and Moderna Inc.—both shown to be more than 90% effective—and the private-sector doctors are angry at being given what, in their view, is a second-class shot. “It’s not that we are acting like spoiled children,” says Paolo Mezzana, a plastic surgeon who’s the spokesperson for a group of about 3,500 private specialists. “We are not against AstraZeneca for the sake of it, but we know that with their vaccine it takes longer to get a complete immunization. We are not class B doctors.”
Rejection of its vaccine in Europe is the latest in a string of problems for Astra. After a bitter public fight between the company and the European Union over supply shortages in January led regulators to tighten controls on exports outside the Continent, the bloc now faces issues over consumer acceptance for the doses it does have. Following a study showing efficacy was considerably reduced against a variant first identified in South Africa, the rollout of Astra’s shot there was temporarily halted. A lack of data on its effectiveness in older adults and questions about optimal dosing intervals haven’t helped.
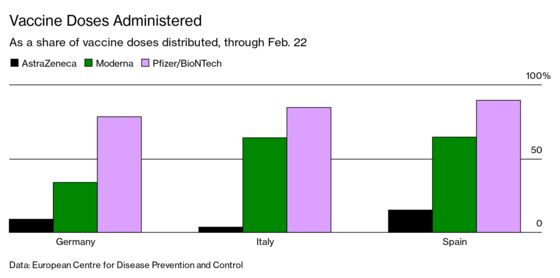
The view of the vaccine as a lesser product is particularly problematic given how key it is to global immunization efforts. Astra Chief Executive Officer Pascal Soriot has said the company plans to make as many as 3 billion doses of the vaccine available this year. Unlike the messenger RNA vaccines from Moderna and Pfizer and its German partner BioNTech SE, which both have to be transported and stored at frozen temperatures, the Astra-Oxford shot just needs to be refrigerated—making it easier to distribute in less-developed areas. Astra is also providing it at no profit during the pandemic, and for low- and middle-income countries in perpetuity, making it one of the most accessible shots. “A combination of mistakes and bad luck, compounded by some unhelpful media coverage and comments by senior politicians, have undermined confidence in this vaccine,” says Martin McKee, professor of European public health at the London School of Hygiene & Tropical Medicine. “Once trust is lost, even if unjustifiably, it is very difficult to restore.”
Since Astra and Oxford announced their initial results last year, data on the vaccine’s effectiveness in preventing symptomatic infection have ranged from 60% to 90% because of different dosing amounts and regimens in the trials. An analysis published in February found the vaccine was 81% effective with a three-month gap between two shots. But the European Medicines Agency puts the efficacy at 60%, based on the trial data it used to approve it.
While the vaccine was approved for all adults by the EMA in January, at least 10 EU countries haven’t cleared it for use in people over 65 because of a lack of data for that population. French President Emmanuel Macron called the shot “quasi-ineffective” for people in that age group. Responding to a lack of European confidence, German Health Minister Jens Spahn said on Feb. 20 that people who receive the Astra vaccine would be able to get a different shot later if there were enough supplies. As of Feb. 22, only 13% of the almost 1.5 million Astra doses delivered to Germany had been administered, partly fueled by hesitancy about the shot.

U.S. data from trials of about 30,000 people expected in the coming weeks could answer some of the questions surrounding the vaccine. About 24% of the participants are older than 65, which should provide sufficient information on the protection it offers older adults. Still, the trials have been run on a four-week dosing regimen, shorter than the 12-week gap the developers say is optimal. An FDA decision on authorization is expected as early as April.
The criticism started in September when global trials of the shot were paused after a U.K. volunteer suffered unexplained neurological symptoms. The British study resumed less than a week later, but the pause stretched to almost seven weeks in the U.S. While such events are common in trials, the developers were accused of a lack of transparency over the piecemeal disclosure of information.
In November, when the partners reported initial data for the vaccine, there was more confusion. Because of a manufacturing error, one group of participants had received a lower first dose, which produced an efficacy reading of 90%, compared with 62% for those getting two standard doses. Astra and Oxford said this produced average efficacy of 70%.
Plans to run additional trials to clarify the number were later walked back after further analysis suggested the discrepancy could have more to do with the dosing interval than the amount. In January, Soriot said the company would conduct a global study on the optimal interval. A week later, Astra executive Mene Pangalos said the drugmaker probably wouldn’t. Real-world data from places such as the U.K., which has recommended a dosing gap of 4 to 12 weeks, will likely provide answers more quickly than completing new trials.
A spokesman for Astra highlighted the analysis published in February, which showed the vaccine is more than 70% effective after one dose and more than 80% after two, using a three-month dosage interval.
Despite the lack of clarity, one important statistic has remained high—the Astra shot’s level of protection against severe disease, hospitalization, and death. Data taken from hundreds of thousands of vaccinated people in Scotland the week of Feb. 22 found the vaccine reduced hospitalization by 94%.
The efficacy of the shot “has not been clearly conveyed, with different numbers relating to different schedules, different intervals,” says David Salisbury, former director of immunization at the U.K.’s department of health. But “the takeaway that needs to be reinforced is that AstraZeneca vaccine recipients are saved from severe disease and death, and that is the most important aspect.”
The threat of viral variants has further plagued the vaccine. Oxford said in February that the shot provides as much protection from symptomatic infection against a key variant first identified in the U.K. as it does against the original strain. But data from the Oxford trial’s South African arm showed the shot had only 22% efficacy against mild and moderate illness from the dominant variant there.
There is no data on whether the vaccine provides protection against severe disease from that variant. A top adviser to the South African government proposed the week of Feb. 15 that the country give doses to 100,000 people as a way to determine whether it’s effective in curbing hospitalization.
Despite its setbacks, the Astra-Oxford vaccine is set to play a valuable role globally, says Anna Bezruki, a researcher at the Global Health Centre at the Graduate Institute of International and Development Studies in Geneva. “We don’t have many tools right now,” she says. “It could not only save lives but reduce a lot of strain on health-care systems.” —With Naomi Kresge and Tim Loh
©2021 Bloomberg L.P.