Two Surprising FDA Rejections Raise Alarms for Biotech Investors
Two Surprising FDA Rejections Raise Alarms for Biotech Investors
(Bloomberg) -- The Food and Drug Administration’s rejection of a pair of medicines that Wall Street expected to sail by regulators raised concerns that other upcoming drug decisions could meet a similar fate.
Setbacks for Galapagos NV and Gilead Sciences Inc.’s rheumatoid arthritis treatment and BioMarin Pharmaceutical Inc.’s hemophilia A gene therapy turned up the heat for biotechnology investors betting on companies with key decisions in the coming months. Shares fell as Biogen Inc.’s experimental Alzheimer’s disease drug and Bristol Myers Squibb Co.’s medicines acquired from Celgene Corp. were seen among those at risk.
“I’m getting the question a lot about how the FDA had been so flexible for recent history and are they coming to a turning point where they’re rejecting applications more freely,” Baird analyst Brian Skorney said by phone. Without seeing the agency’s communication with companies, “it’s hard to tell” how much readthrough there might be to other drugs under review, he said.
Biogen fell 2.8% on Wednesday, also weighed down by news of new generic competition for its multiple sclerosis drug. That puts even more pressure on aducanumab, an experimental therapy for Alzheimer’s that has an FDA decision deadline of March 7.
Bristol Myers’s lisocabtagene maraleucel has a target decision date of Nov. 16 in large B-cell lymphoma and its application with partner Bluebird Bio Inc. for idecabtagene vicleucel in multiple myeloma was re-filed with U.S. regulators last month. Approval of liso-cel by Dec. 31 and ide-cel by March 31 would mean a $9 payout for holders of the contingent value right that was issued in the Celgene deal. Shares of the CVR fell as much as 2% on Wednesday to $2.88.
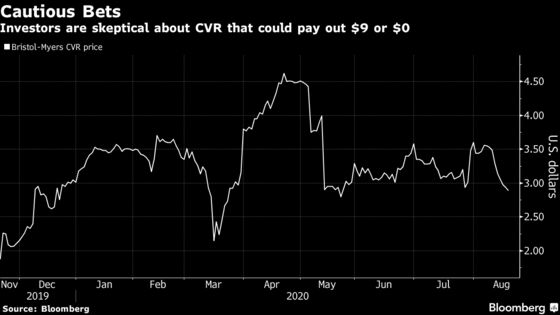
Jared Holz, a health-care strategist at Jefferies, said he doesn’t think the Gilead and BioMarin setbacks suggest “some sort of sea change or trend with respect to how the FDA handles these drug approvals.” The agency is seeking more data on both drugs, which analysts expect will delay approval by about a year for Gilead and Galapagos and closer to two years for BioMarin.
In BioMarin’s case, Holz said the availability of other drugs to treat hemophilia may give the FDA more leeway to take things slowly. The agency may also be signaling a higher bar for approvals in the new class of medicines known as gene therapies, he said.
For Biogen, the lack of approved treatments in Alzheimer’s could prove to be a key differentiator when it comes time for the FDA decision. Bristol-Myers’s liso-cel and ide-cel would compete with old and new medicines.
Other recent signals that the FDA may be growing more cautious include a New York Times report that the agency has placed the authorization for blood plasma as a Covid-19 treatment on hold as it awaits more data.
©2020 Bloomberg L.P.