Trump-Touted Drug Lives On as Covid Therapy Despite Trial Flops
Trump-Touted Drug Lives On as Covid Therapy Despite Trial Flops
(Bloomberg) -- Hydroxychloroquine, the antimalarial drug that former President Donald Trump touted as a “game changer” in the fight against Covid-19, is still being prescribed by physicians in the U.S. though it has proven to be ineffective against the virus in clinical trials.
Concern is growing that patients are at risk of harm because physicians continue to prescribe hydroxychloroquine over other potentially life-saving Covid treatments. In June, the Food and Drug Administration revoked the emergency use authorization for hydroxychloroquine “in light of ongoing serious cardiac adverse events and other serious side effects.” The potential benefits of the drug no longer outweigh the known and potential risks for the authorized use, the agency said in a statement.
“I would say if it’s not malpractice then it’s certainly close,” said William Haseltine, a former Harvard Medical School professor and a pioneering HIV researcher who now chairs Access Health International, a health equity think tank. “It’s unfortunate. The patients that are receiving [hydroxychloroquine] are not receiving benefit. It’s not particularly toxic, although it is toxic in some cases for people with heart problems -- so it could harm those people.”
Patients across the country are still seeking the drug and some doctors prescribe it off-label, meaning for unapproved purposes. Some physicians are convinced that hydroxychloroquine, in combination with other drugs, could help prevent mild Covid symptoms from worsening. From their perspective, it has been on the market for decades and has been used off-label in various ways by respected members of their field.
While off-label prescribing is common and legal, trial after clinical trial has shown that treatment of Covid-19 with hydroxychloroquine shows no clinical benefit -- at any stage of the disease. The FDA declined to comment.
Still Prescribing
George Smith, a family doctor who runs a solo practice in Covington, Georgia, said he has prescribed hydroxychloroquine to about 160 Covid-19 patients since the virus hit his community in March 2020. Many came to him because their own physicians wouldn’t treat them with the drug.
He resigned from a job as a nursing home physician after the medical director refused to allow him to treat patients with hydroxychloroquine.
“There have been so many people who have died from Covid needlessly because of government obstruction -- it’s a crime, it’s such a shame,” Smith said in a phone interview. “I look at it this way: I swore the Hippocratic oath when I finished medical school to treat my patients the best way I know how -- and so that’s what I’ve done. If they come and take my license, then they come and take my license. But I’m not going to let somebody die over some stupid government regulation.”
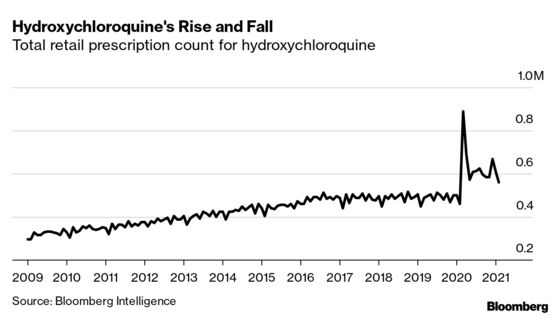
The FDA authorized the emergency use of hydroxychloroquine in March 2020. A week later Trump endorsed the drug as a potential Covid-19 treatment and the government stockpiled millions of doses. After Trump promoted the drug in March, monthly prescriptions jumped 93% to 890,000.
While demand for the drug has tailed off since then, it is still significantly higher than at the start of the pandemic -- in February 2021, there were 560,000 hydroxychloroquine prescriptions written, 22% higher than a year earlier. It’s difficult to predict how many of these were off-label, though researchers estimate that off-label versions make up between 12% and 38% of all prescriptions in the U.S, according to a recent report by the Congressional Research Service.
The rush in 2020 led to shortages of the drug for patients with lupus, rheumatoid arthritis, and other autoimmune diseases, for which hydroxychloroquine has proven to be an effective treatment. As a result, 18 states issued emergency restrictions that prohibited prescribing and dispensing of the drug for Covid-19 use. Other states didn’t go as far but most released guidance discouraging inappropriate prescriptions.
As of March 2021, restrictions remain in place in only six of the 18 states. Mike Donnelly, a spokesperson for the Lupus Foundation of America, says the non-profit group has not heard of patients experiencing difficulties accessing hydroxychloroquine since last year. In March a World Health Organization panel warned that hydroxychloroquine should not be used to prevent infection with Covid-19.
Patient Requests
A conservative non-profit group says patients still want the drug. The Association of American Physicians and Surgeons compiles a list of doctors who are willing to prescribe hydroxychloroquine. Jane Orient, the group’s executive director, says many patients use telehealth to get a doctor, and they can get their prescriptions filled at an out-of-state pharmacy if they don’t have a local pharmacist who wants to do so.
“In our newsletter each month we ask our members to list themselves if they are willing to accept new patients for this purpose. Many physicians don’t want to be public about this because there are some concerns about complaints going to the medical board, which are, at best, a very stressful and expensive time-consuming hassle,” Orient said.
The AAPS, which is also known for opposing federal vaccine mandates, filed a lawsuit against the government in June 2020 citing the “irrational interference by the FDA with timely access to hydroxychloroquine.” The group wants an injunction against the FDA’s decision in March 2020, which prohibits the use of the donated hydroxychloroquine except for already hospitalized patients.
They’ve also created a 26-page, home-based Covid treatment guide for patients, which advises taking a combination of anti-viral medicines at the onset of symptoms.
Peter McCullough, a professor of medicine at Texas A&M University’s College of Medicine and an AAPS member, considers himself a firm believer in using hydroxychloroquine in combination with other drugs to treat Covid patients. “The biggest mistake in people reporting and even in the scientific investigations is to try to declare or not declare whether hydroxychloroquine works -- that’s a fool’s errand because we use drugs in combination,” he said.
He said he’s prescribed a multiple-drug regimen that includes hydroxychloroquine for about 100 high-risk Covid patients and advised “hundreds more” doctors who then went on to treat thousands more patients, he said.
A few other drugs that have shown greater effectiveness against Covid-19 have since gained authorization, including Gilead Sciences Inc.’s remdesivir and the antibody cocktail from Regeneron Pharmaceuticals Inc. that Trump touted after he was diagnosed with the disease. Ridgeback Biotherapeutics and Merck & Co. have partnered to develop an oral antiviral treatment, molnupiravir, and are encouraged by the findings so far.
Myron Cohen, associate vice chancellor for global health and medical affairs at the University of North Carolina School of Medicine at Chapel Hill, says this illustrates a broader medical issue that is not unique to Covid-19 -- it’s hard to move people away from their own anecdotal evidence.
“Hydroxychloroquine was just filling a vacuum,” Cohen said. “In the long run, health providers are going to accede to drugs that have been proven to work.”
©2021 Bloomberg L.P.