Flood of July Vaccine Data Starts on Pfizer Positive Note
Flood of July Vaccine Data Starts on Positive Note From Pfizer
(Bloomberg) -- Exhausted health-care investors who might feel like they haven’t had a day off since March aren’t going to catch a break this summer, as a barrage of new data on Covid-19 vaccines and treatments are expected in the third quarter.
With more than 10 million confirmed cases around the globe and over half a million deaths from Covid-19, drug and vaccine makers are under increasing pressure to deliver.
For the third quarter “it’s all about vaccines,” according to Umer Raffat, an analyst at Evercore ISI. Results from Moderna Inc., AstraZeneca Plc and Pfizer Inc.’s partnership with BioNTech SE will be among the most watched events in the quarter, Raffat said in a phone call on Friday.
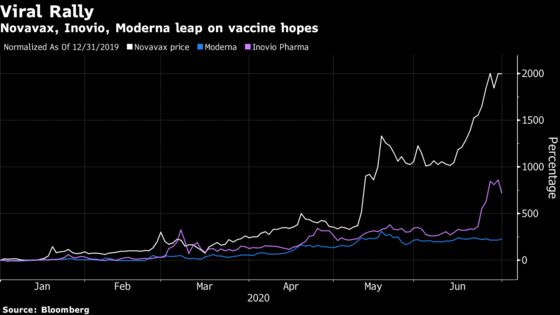
Indeed, results from Pfizer and BioNTech kicked off the quarter with a bang after a study in 45 healthy adults drove a high level of antibody responses in those getting two shots. Shares of Pfizer rallied as much as 5.6% on Wednesday while BioNTech jumped as much as 19%. Other vaccine developers fell on the news, including Moderna which slid 7.9% and Inovio Pharmaceuticals Inc., which dropped 32%.
Vaccines
A more detailed look at Moderna’s messenger RNA vaccine is expected to appear in a peer-reviewed journal soon, which may answer some lingering questions over the level of antibodies that the vaccine produced. A final-stage study of Moderna’s vaccine in 30,000 people is set to begin this month. Investors will also be looking for results in July from a vaccine AstraZeneca is developing with Oxford University.
Investors may also get a first look at a messenger RNA product from BioNTech and Pfizer sometime in the third quarter. The companies are simultaneously developing four different vaccine candidates and one could enter the final stage of testing later this summer.
Johnson & Johnson will start the first stage of testing of its adenovirus-based vaccine in the latter half of July.
In August, closely-held Clover Biopharmaceuticals plans to reveal safety and immunogenicity results for its vaccine, which uses immune-boosting technology from GlaxoSmithKline Plc and Dynavax Technologies Corp.
Much of the focus is on larger companies, and smaller players like Novavax Inc. and Inovio have faced some skepticism on the back of day trader-driven rallies. Even so, Raffat notes that it’s not impossible that the smaller companies will find success. Novavax plans to report results for the first stage of human testing on its vaccine in July.
Antivirals
Doctors are also looking for more treatment options after some early success with the generic steroid, dexamethasone, and Gilead Sciences Inc.’s remdesivir infusions. Additional results for remdesivir from an open-label study may come out over the summer.
Merck & Co. is also working with collaborator Ridgeback Biotherapeutics on EIDD-2801, an oral antiviral drug that may start late-stage studies in thousands of patients in July, according to Morgan Stanley. Since it’s a pill, EIDD-2801 could be taken immediately after diagnosis, analyst David Risinger wrote. He sees “blockbuster potential” for the medicine with a potential approval before the end of the year if larger trials are successful.
Antibodies
Antibody results from Eli Lilly and Regeneron Pharmaceuticals Inc. may also grab headlines in the third quarter. The pair are in the lead developing so-called neutralizing antibodies that can mimic immune responses to the virus and stop the deadly infection. Such treatments “could potentially ‘bend the curve’ of Covid-19 outcomes if they prove effective in treating early or mid-stage infections,” said Steven Seedhouse, an analyst with Raymond James.
Given the precedent set by remdesivir, these medicines could breeze through regulatory hurdles if they show “any clinical benefit with an acceptable safety profile,” Goldman Sachs analyst Terence Flynn said. While antibody treatments have to be infused and aren’t expected to drive massive sales for the manufacturers, Flynn is optimistic about their prior success in Ebola and the relative ease in manufacturing.
They might work both as a treatment against existing infection and as a preventative, “including in populations that often don’t respond well to vaccines,” Flynn wrote in a note to clients.
Results for Lilly’s LY-CoV555 were expected by the end of June, according to Goldman. Regeneron has said it could produce an antibody cocktail by the end of the summer.
©2020 Bloomberg L.P.