Seres Surges 611% After Colon Therapy Meets Goal in Study
Seres Surges 611% After Colon Therapy Meets Main Goal in Study
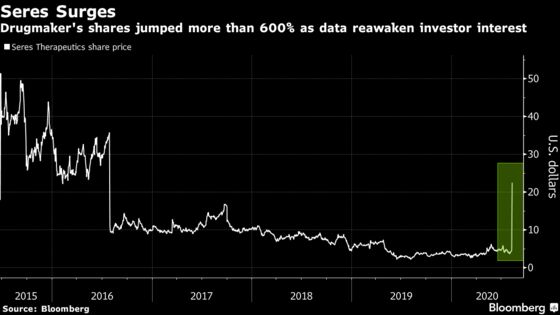
(Bloomberg) -- Small-cap drug developer Seres Therapeutics Inc. soared sevenfold to the highest in four years Monday after a study for its drug in patients with a bacteria-driven colon infection was heralded as a landmark for both the company and the entire microbiome space.
The positive results from the pivotal trial -- dubbed “Ecospor III” -- showed the effectiveness of its lead drug SER-109 for recurrent C. difficile infection which causes damage to the lining of the colon. The results “move the needle” for the company and the entire microbiome therapeutics space, Jefferies analyst Chris Howerton said in an email.
Seres said the drug cut the recurrence of infection by about 30% within eight weeks of treatment compared with those receiving a placebo. Of patients that received SER-109, 88.9% showed a sustained response, the company said in a statement.
Shares of the Cambridge, Massachusetts-based company shot up by a record 611% at 10:22 a.m. in New York, fueling the stock to the highest level since July 2016. The rally came on more than 107-times the average three-month daily volume and prompted at least seven trading halts. Seres, which went public five years ago, had shed 74% of its value through Friday’s close. It’s now up about 12% since the IPO.
The ultimate label will be important with toxin testing required, however, the drug’s effectiveness and the number of patients could make its targeted market a $1 billion opportunity, Jefferies’s Howerton said. The data also should reawaken interest in peers Evelo Biosciences Inc. and Synlogic Inc. -- and could open “up the door for broader public companies on the market through” initial public offerings, he continued.
The drug was well tolerated and had a safety profile deemed similar to the placebo with the most common side effects of flatulence, abdominal distention and abdominal pain being balanced between both groups of patients. Seres believes the data support a drug filing with U.S. regulators and said it plans to request a meeting to get the drug breakthrough status soon.
“These results are the most compelling we have seen for any microbiome restoration therapy to date, and represent a major vindication for Seres’ platform technology and rational approach to study design,” wrote Oppenheimer analyst Mark Breidenbach who raised his price target to a Street-high $30 from $7.
©2020 Bloomberg L.P.