Sarepta Climbs as Gene Therapy Shows Promise in Early Study
Sarepta Climbs as Gene Therapy Shows Promise in Early Study
(Bloomberg) -- Sarepta Therapeutics Inc.’s bulls may get some validation after the company’s experimental gene therapy for a rare type of muscular dystrophy improved physical fitness in a trio of patients.
Updated data for the experimental compound showed patients with an inherited form of Limb-girdle muscular dystrophy reaped functional benefits in several areas after receiving SRP-9003, with a pair of teenagers posting gains in their ability to move when they typically would have experienced declines. Analysts had stressed that promising results could help the company shake-off a recent rout that wiped out $6 billion in market value as questions about the company’s drug pipeline mounted.
The results for the three patients showed a 1.3 second to 10.9 second improvement in the time it took to run 100 meters and a 0.1 second to 0.9 second benefit in their ability to climb four stairs. That contrasts with Evercore ISI’s assessment that “any improvement or even stabilization on the various functional endpoints could be interpreted as positive drug effect by the Street.”
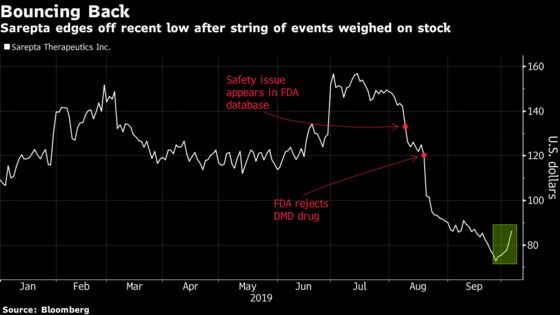
Shares of the Cambridge, Massachusetts-based drugmaker rose as much as 9.7% Friday, on pace to extend their longest winning streak since February. The rally comes after the stock closed near a 17-month low on Sept. 26 as the broader market churned.
“On every single functional endpoint, every patient is improving,” Chief Executive Doug Ingram said, expressing confidence in the program. Even a stabilization in measures such as the North Star Assessment for Dysferlinopathy (NSAD) would have been a great indicator, Ingram continued, though the number of patients treated is small and the results are early.
Production of an enzyme that the muscle releases when it’s being damaged, known as CK, fell an average of 82% at nine months, slipping slightly from the 90% reduction seen previously. The company executives attributed the incremental decline to increased patient activity and said they remained focused on the broader benefit as another promising sign. The initial results, shown in February, fueled an 8.3% rise to a five-month high.
“The take home message is that on every single measure, patients improved across the board,” Louise Rodino-Klapac, Sarepta senior vice president of gene therapy, said by phone.
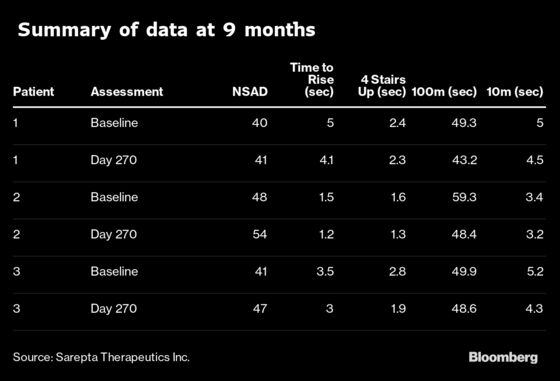
The patient benefit looked “highly impressive” to RBC analyst Brian Abrahams, who wrote in a note there was “little to nitpick” from the data. Friday’s news is “an important step” toward starting to turnaround sentiment for the company and Abrahams advised investors buy into the stock’s strength.
The company plans to infuse additional patients with a higher dose of SRP-9003 so it can compare the benefits before selecting which to strength to take into a larger study.
“We want to dose the patients as soon as possible,” Ingram said. “Our goal is to try and get them dosed this year if at all possible.”
Skeptics of Sarepta’s Limb-girdle program have highlighted that the natural history of the disease isn’t well known, limiting the ability to compare benefits using data from just three patients. There have also been concerns that the two 13-year-old patients are likely in the declining phase of muscle function, while the four-year-old is still growing. That could make the improvements even more difficult to interpret.
No new safety signals were seen in the updated data, supporting plans to test a higher dose of the gene therapy. It’s worth noting that there are no current treatment options for the rare, progressive disease, which leads to weakness and atrophy of the muscles supporting the hips and shoulders.
Despite the promising results, investors are likely to remain laser-focused on mid-stage data from the company’s Duchenne muscular dystrophy gene therapy, with data expected next year. In addition to patients with Limb-girdle disease-2E, Sarepta has four other programs for the broader limb-girdle patient population.
To contact the reporter on this story: Bailey Lipschultz in New York at blipschultz@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Michelle Fay Cortez, Cristin Flanagan
©2019 Bloomberg L.P.