Remdesivir Averts Hospitalization in Study of High-Risk Patients
Remdesivir Averts Hospitalization in Study of High-Risk Patients
(Bloomberg) -- Early treatment with Gilead Sciences Inc.’s remdesivir kept high-risk Covid-19 patients out of the hospital in a late-stage international trial that supports administering the medicine to individuals recovering at home.
A three-day course of the injectable antiviral, also known as Veklury, was associated with an 87% reduction in hospitalizations in a randomized, placebo-controlled trial involving 562 patients, Gilead said in a statement Wednesday. The data are slated for release at a virtual medical meeting on Sept. 29.
Almost a year after remdesivir became the first medication approved by the U.S. Food and Drug Administration for treating Covid, sales have fallen amid widening use of immunizations to prevent severe disease and alternative therapies, such as monoclonal antibodies.
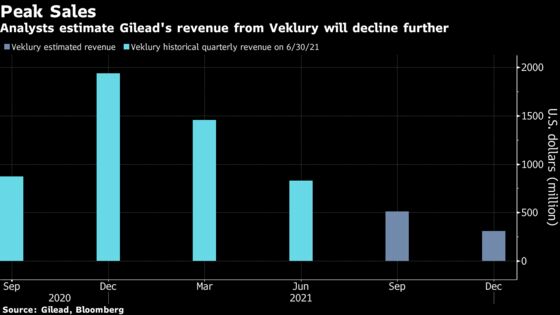
The latest finding on remdesivir adds to evidence that antivirals can avert disease progression if given within days of a SARS-CoV-2 infection. Investigators found nurses can safely administer the infusions, which typically take 30 to 60 minutes, in patients’ homes or residential-care facilities, said Joshua Hill, an infectious disease physician at the Fred Hutchinson Cancer Research Center in Seattle, who will present the data next week.
“The shorter-term dosing just for three days and being able to give it in a variety of different practice settings and even in patients homes, I think is really exciting,” Hill said in a Zoom interview.
Gilead sponsored the trial. It was stopped in April 2021 prior to fulfilling enrollment targets, mostly because patients vaccinated against Covid-19 and those receiving monocloncal antibody therapies were ineligible to participate.
The study enrolled non-hospitalized patients within a week of developing Covid symptoms. Participants were aged 60 or older or had one or more risk factors known to increase the likelihood of developing severe illness.
Patients were randomly selected to receive either three doses of remdesivir over consecutive days or a corresponding placebo regimen. The trial found those on remdesivir were 81% less likely to attend a Covid-related medical visit. None of the participants died in the four weeks during which they were followed.
Both remdesivir and monoclonal antibody treatments offer a similar level of benefit and could be given in combination to high-risk Covid patients, such as transplant recipients, and those receiving chemotherapy and renal dialysis, who may not receive adequate immune protection from vaccination, according to Hill.
“Is that even better in a particularly high-risk group? I think it very well could be, and there’s a lot of precedent for that,” he said. “I think that’s probably part of the future.”
©2021 Bloomberg L.P.