Red-Hot Biotechs to Take Spotlight at Blood Disorder Meeting
Red-Hot Biotechs to Take Center Stage at Blood Disorder Meeting
(Bloomberg) -- High-flying drug developers that have surged in the face of the ongoing coronavirus pandemic will be in focus starting this weekend at the year’s last big medical meeting, putting surging stocks under the microscope for investors who have cheered massive gains.
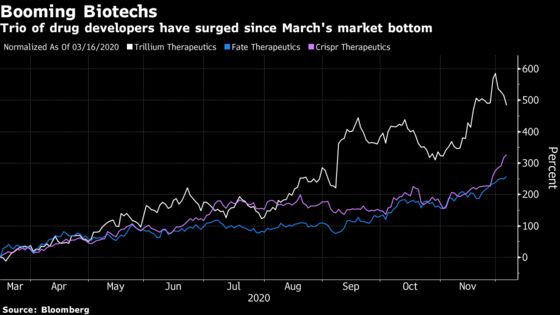
Updated results from Trillium Therapeutics Inc., which has boomed 1,550% this year, will be among those most closely watched by Wall Street at the American Society of Hematology (ASH) meeting that kicks off Saturday. Analysts are also highlighting new test results from Crispr Therapeutics AG and its partner Vertex Pharmaceuticals Inc.’s gene editing platform, as well as Fate Therapeutics Inc.’s cell therapy. Crispr surged 4.2% Friday to another record and shares in Fate have more than tripled this year.
The meeting could provide another leg of growth for the Nasdaq Biotech Index, which has gained 23% this year compared with the S&P 500’s 14% rise.
The options market shows partners Crispr and Vertex are primed to see the most outsized moves in the week following their presentation. An analysis of Crispr options set to expire on December 11 shows its shares are expected to move 12% by then and implied volatility is elevated at about 95, compared to a three-month average of 66. Contracts expiring on Dec. 11 in Vertex show shares are expected to move by 4.4% with implied volatility trading around 36 versus a three-month average of about 50.
Wendy Lam, portfolio manager with Franklin Templeton, is looking forward to the event as companies continue to advance medicines that can combat blood disorders. “We haven’t seen the pandemic impact innovation across the sector; we’re excited about gene therapy and gene editing,” she said. Lam highlighted updates from cell therapy companies that she sees as “being an area of focus for every ASH going forward.”
Wall Street will be looking to better understand the growing field of medicines targeting a protein linked to cancer, known as b-cell maturation antigen (BCMA). Updates for CAR-T cancer programs and bispecific candidates will pit the likes of Johnson & Johnson against Amgen Inc. and others.
The group of programs leaves the BCMA field “quite crowded,” according to analysts from Robert W. Baird, who highlighted data from J&J and Regeneron Pharmaceuticals Inc. They warned that there is limited differentiation among the researchers, which “could lead to a highly fragmented, unattractive commercial market.”
Here are some of the presentations analysts and investors have highlighted (all New York time):
Crispr Therapeutics and Vertex Pharmaceuticals
- Updated results from Phase 1/2 clinical trials of the investigational CRISPR/Cas9 gene-editing therapy CTX001 in patients with sickle cell disease and beta thalassemia on Dec. 6 at 12:30 p.m.; companies to host call and webcast on Dec. 9 at 8 a.m. to discuss the data
Trillium Therapeutics
- Updated data from study of TTI-622 in patients with advanced relapsed or refractory lymphoma on Dec. 5 at 10 a.m.
- Updated results from study of TTI-621 in patients with relapsed or refractory hematologic malignancies on Dec. 7 at 3 p.m.
- Company will host a call to review the data on Dec. 7 at 4:30 p.m.; 236-389-2162, password: 3169183
Allogene Therapeutics
- Results from Phase 1 study of ALLO-715 in relapsed/refractory multiple myeloma on Dec. 5 at 12:30 p.m.; management will host a webcast at 2 p.m. that day to discuss the results
Forma Therapeutics
- Results for Phase 1 study of FT-4202 in sickle cell disease with oral presentation Dec. 7 at 5 p.m.
Fate Therapeutics
- Data for FT596, an off-the-shelf CAR NK cell therapy, in relapsed/refractory B-cell lymphoma on Dec. 6
IGM Biosciences
- Phase 1 data of IGM-2323 in relapsed or refractory non-Hodgkin’s lymphoma on Dec. 5 with company call and webcast following presentation at 2 p.m.
Uniqure
- Data from Phase 3 “Hope-B” pivotal trial of etranacogene dezaparvovec gene therapy in hemophilia B on Dec. 8 at 11:45 a.m.; management will host a webcast at 5 p.m. to review the data
Legend Biotech and Johnson & Johnson
- Phase 1b/2 data for ciltacabtagene autoleucel, a BCMA-directed CAR-T cell therapy for patients with relapsed or refractory multiple myeloma on Dec. 5 at 3 p.m.; Legend hosting a call to review the data on Dec. 7 at 7 p.m.
Constellation Pharmaceuticals
- Phase 2 data from study of CPI-0610 as a monotherapy in advanced Myelofibrosis patients refractory/intolerant to JAK inhibitors on Dec. 5 at 10 a.m.
- Phase 2 data from study of CPI-0610 as an add-on to Incyte’s Jakafi in advanced Myelofibrosis patients with suboptimal response on Dec. 5 at 11:45 a.m.
- Company hosting call to discuss the two presentations and posters on Dec. 7 at 8 a.m.
Gilead Sciences
- Long-term survival and gradual recovery of B Cells in patients with refractory large B cell lymphoma treated with axicabtagene ciloleucel (axi-cel) on Dec. 5
- Data on magrolimab in patients with previously-untreated acute myeloid leukemia who cannot undergo treatment with intensive chemotherapy, including patients with TP53-mutant AML on Dec. 6
- Analysis of Phase 2 “Zuma-5” study of axi-cel in patients with relapsed/refractory indolent non-Hodgkin lymphoma on Dec. 7 at 4:30 p.m.
Amgen
- Updated data from Phase 1 dose escalation study of AMG 701 in patients with heavily pre-treated relapsed/refractory multiple myeloma on Dec. 5 at 1 p.m.
Regeneron Pharmaceuticals
- Data from REGN5458, a bispecific antibody, in patients with relapsed/refractory multiple myeloma on Dec. 5
- Data from Phase 1 trial of REGN1979 in patients with highly refractory B-cell non-Hodgkin lymphoma including patients refractory to CAR-T therapy on Dec. 6
- Company hosting a call on Dec. 7 at 4:30 p.m.
Syndax Pharmaceuticals
- Data for Phase 1 trial of axatilimab in chronic graft versus host disease on Dec. 6 at 1:45 p.m.; company to host a call after the presentation at 2 p.m.
Bluebird Bio
- Updated results from patients in Group C of Phase 1/2 “HGB-206” study of LentiGlobin for SCD gene therapy on Dec. 7 at 4:30 p.m.
- Updated safety and efficacy results from the Phase 1 “CRB-401” clinical study of Bristol Myers-partnered idecabtagene vicleucel (ide-cel) in patients with relapsed and refractory multiple myeloma on Dec. 5 at 1 p.m.
- Data from ongoing Phase 1 “CRB-402” clinical study of bb21217 in patients with relapsed and refractory myeloma on Dec. 5
- Subgroup analyses of pivotal “KarMMa” study of ide-cel in high-risk newly diagnosed Multiple Myeloma on Dec. 5
- Company hosting a call to discuss the data on Dec. 7 at 7 p.m.
Seagen and Takeda
- Five-year update for Phase 3 “Echelon-2” study of Adcetris with chemo as a first-line treatment of patients with CD30-positive Peripheral T-cell lymphoma on Dec. 5
- Five-year update for Phase 3 “Echelon-1” study of Adcetris with chemo as a first-line treatment of Stage III/IV Classical Hodgkin lymphoma on Dec. 7
Incyte
- Phase 3 “Reach3” study of Jakafi vs best available therapy in patients with steroid-refractory/steroid-dependent chronic graft-vs-host disease on Dec. 5
- Analyses from Phase 2 study of Tafasitamab combined with Lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma on Dec. 7
- Phase 1b data from trial of Tafasitamab or Tafasitamab and Lenalidomide in patients with newly diagnosed diffuse large B-cell lymphoma on Dec. 7
©2020 Bloomberg L.P.