The Race to Supply Flu Shots Before Brexit Is On
Race to Supply Flu Shots Was Already Stressful. Now Add Brexit.
(Bloomberg) -- Manufacturing flu shots and delivering them to the masses is complex any year. Brexit takes it to an entirely new level.
Ken Lim can attest to that. He has overseen efforts at vaccine maker Seqirus to brace for the possibility of the U.K. leaving the European Union without a deal and supplies being disrupted at the border. That has meant booking space on airplanes to cut the risk of delays and opening an office in Amsterdam to clear any new regulatory hurdles. The cost to prepare for a worst-case scenario has climbed to more than 10 million pounds ($12 million).
“There were some people who thought, well, that’s not going to happen,” said Lim, head of strategy at Seqirus. “Now we’re very grateful for having made that decision. But it has come at what for us is a considerable cost.”
The prospect of a no-deal divorce has intensified concern about food and medicine shortages when the Oct. 31 Brexit deadline passes. Flu season gets under way at the same time, ratcheting up pressure on vaccine manufacturers and the country’s National Health Service. The shots are crucial in controlling a virus that kills thousands of people in England each year.
The health-care industry has been preparing for a disorderly departure from the EU for more than two years. When it comes to flu vaccines, most shipments this season are due to arrive in the U.K. before the end of the month. Regardless of how Brexit plays out, however, the companies and their intricate supply chains have already come under strain.
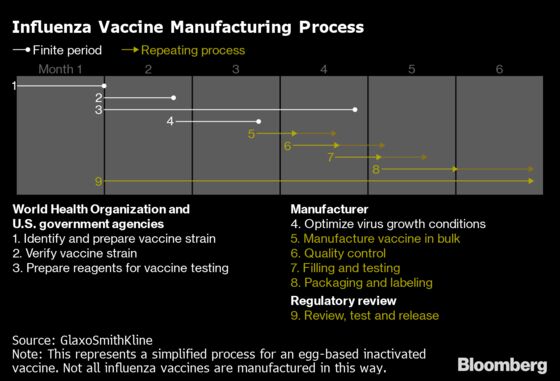
Getting the shots from the plant to the patient is complicated. Another supplier, French drugmaker Sanofi, has described the annual battle against the unpredictable and constantly evolving foe as a “race against the clock.”
For the northern hemisphere, it begins in February with a World Health Organization recommendation on which strains of the virus to include in the vaccines. The shots are then produced, mostly by growing inactivated viruses in chicken eggs. Scientists have relied on this process for decades, though some companies are shifting to technology that may offer better protection.
Crossing Border
Seqirus makes one of its vaccines, Fluad, in Liverpool, England and sends it to Spain and Belgium for packaging. From there, the shots come back across the border and are distributed to U.K. hospitals and pharmacies. The company, the biggest supplier to Britain, is delivering a total of almost 12 million doses to the country.
This time, Brexit forced Seqirus to take extra measures, including securing air freight capacity. To deliver medicines to the EU, companies need to oversee their testing and release from an office within the bloc, so Seqirus also had to build a facility in the Dutch capital to satisfy the rules once the U.K. leaves.
“It has taken a really significant amount of work to get to this point,” Lim said. “We simply couldn’t take the risk of not being prepared.”

Sanofi makes its flu vaccines in Val de Reuil, more than 60 miles northwest of Paris, before sending them on to the U.K. and other destinations. Britain’s looming exit pushed the drugmaker to come up with alternatives to the busy port of Dover, England, to avoid possible congestion. The French company, which is supplying about 7 million doses to Britain, started bringing the shots to another port, Newhaven, and arranged to fly the vaccine in if necessary.
The company’s Brexit costs have run into the millions of euros.
“We’re ready to go,” said Hugo Fry, its U.K. head.
Seqirus, part of Australia’s CSL Ltd., said it’s on track to make all deliveries by the middle of this month, while Sanofi estimates almost a fifth of its doses are due to arrive after Oct. 31. A WHO delay in identifying the vaccine make-up this year set Sanofi back slightly, Fry said. The supply hurdles have come just as demand for the French company’s flu vaccines in Europe has risen about 20%, he said.
Those vaccines provide a critical defense against a viral infection that can lead to severe illness in high-risk groups, including older adults, young children and pregnant women, sending tens of thousands of patients to already short-staffed hospitals every winter. The shots can avert as many as 625,000 cases of influenza each year in England, CSL estimates.
Protecting Workers
“They not only protect those who are vulnerable, but those who are working with the vulnerable,” said Saffron Cordery, deputy chief of NHS Providers, which represents hospitals. “It’s very important there’s an adequate supply.”

In an August letter to Prime Minister Boris Johnson, a group of doctors led by the Royal College of Physicians expressed worries about Brexit and flu season coinciding and said they’re “simply unable” to reassure patients that health care won’t be hurt by shortages of drugs and devices. The government has warned of potential delays without an agreement to smooth the transition, suggesting in a worst-case assessment that medicines could be especially vulnerable.
Companies have boosted their stocks of medicines to try to make sure that doesn’t happen, ramping up preparations ahead of the original March departure date. The challenge is even greater for flu vaccines and other products that can’t be stockpiled.
The government purchased about 400,000 additional doses of the vaccine for adults in case doctors, pharmacies or hospitals run low and is confident it will have sufficient supplies. Seqirus and Sanofi said they’re prepared, but there’s only so much they can control.
“Where the risk emerges is the unknowns,” Lim said, such as the emergence of “bottlenecks that no amount of contingency planning can fully anticipate.”
To contact the reporter on this story: James Paton in London at jpaton4@bloomberg.net
To contact the editors responsible for this story: Eric Pfanner at epfanner1@bloomberg.net, John Lauerman, Rick Schine
©2019 Bloomberg L.P.