Philip Morris’s By-the-Books E-Cig Approval Now Looks Smart
Philip Morris’s By-the-Books E-Cig Approval Now Looks Smart
(Bloomberg) -- For more than a decade, Philip Morris International Inc. plodded through the painstaking development and rigorous regulatory review of a device people could use instead of cigarettes. IQOS reached $900 million in global sales last year, has 8 million total users and is just entering the U.S.
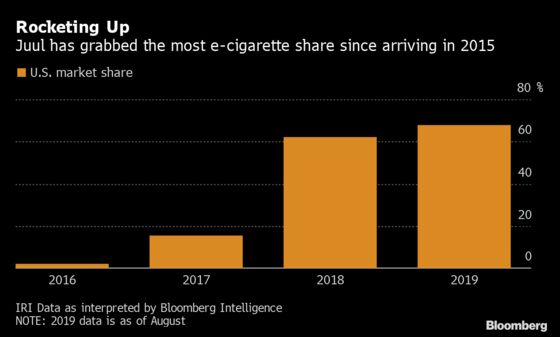
Meanwhile, a San Francisco-based startup unleashed a USB-stick-sized electronic cigarette that took the U.S. by storm, becoming so popular that its name became synonymous with vaping. Juul Labs Inc. exceeded $1 billion in sales last year, has captured more than two-thirds of the domestic electronic cigarette market and seemed destined to dominate the scene for years to come.
As Juul falls from grace amid an escalating public-health crisis heightened by an outbreak of a mysterious lung disease, Philip Morris’s by-the-rules slog is now looking smart. The company’s shares fell 1.1% to $74.43 at 10:02 a.m. in New York after rising 5.2% on Wednesday. Shares of Altria Group Inc., a significant Juul backer, declined 0.9% to $40.20.
“All big tobacco companies have been playing by the book and understand the regulators’ motivations better than many of these upstart, smaller companies like Juul,” said Alicia Forry, an analyst at Investec. “The strategy for all of them has been to do it right.”
Deal Scrapped
Altria, which sells Marlboro cigarettes in the U.S., and Philip Morris, which sells them abroad, declined to comment on why the deal fell apart. But controversy around Juul was widely discussed as the problem. Juul’s CEO stepped down at the same time, and the company said it would halt advertising.
It wasn’t the first sign of trouble: Just as deal discussions became public, talk of banning Juul’s popular flavors intensified, with concerns they were getting a new generation addicted to nicotine. More recently, a health crisis has emerged over the use of electronic cigarettes that have sickened more than 500 people and been blamed for several deaths.
“The current vaping panic in the U.S. was probably the wrong backdrop to finalize a deal,” said Jonathan Fell, principal at London-based investment manager Ash Park, which owns shares in Philip Morris and Altria.
The changes at Juul mark the second time in as many days that a fast-moving startup seeking to shake up a staid, established industry was pushed back on its heels. On Tuesday, the brash CEO of WeWork was forced out of his job after the office-space company scrapped plans for one of the year’s most anticipated IPOs. Juul vowed to upend smoking, only to be undone by the scale of its ambitions and pushed into the arms of the business it once sought to revolutionize.
Good Timing
But the bad news for Juul could be good for IQOS, given it was authorized for U.S. sales in April after a rigorous FDA process that looked at factors like toxicology reports on ingredients and inhalation products. Philip Morris started that process in 2017. It had already asked the FDA for permission to market it with claims it’s less risky than cigarettes -- but the agency has yet to respond. The regulatory rules of the road for companies like Juul are less clear, but it has since said it would go through a similar process. That’s still a ways out, with Juul planning to submit a “premarket tobacco application” by May 2020.
The reversal of fortune came fast. Earlier this year, the prospect for IQOS -- a heat-not-burn device -- wasn’t looking that good, and Wall Street was getting impatient.
On Philip Morris’s fourth-quarter call, it said it missed its full-year net growth target, due almost entirely to lower-than-expected IQOS growth in Japan. At a February conference in New York, as executives talked about next-generation IQOS, one attendee asked if it would outsource production “so you don’t need the big Capex you did” with the earlier model. Philip Morris executives were asked about IQOS’s slow U.S. approval process at every opportunity, and quarter after quarter, they could only offer shareholders a wait-and-see response.
Meanwhile, Juul’s popularity skyrocketed, capturing more than two-thirds of U.S. e-cigarette market share, according to IRI Data as interpreted by Bloomberg Intelligence.
But those investors who thought Philip Morris missed the boat on e-cigarettes in America may be finding that IQOS hits shelves at just the right time -- right as the tide flips on Juul. Philip Morris has since 2008 spent $6 billion to develop IQOS, about half as much as Altria spent to buy a 35% stake in Juul last year.
Altria, which will market IQOS in the U.S. for Philip Morris, is starting U.S. sales first on a small scale, with Atlanta as a test market. And it’s approaching sales with the same careful tack it took with regulators: Anyone coming into a retail location is screened first to see if they are over age 21 -- higher than minimum smoking age of 18 in Georgia -- and also asked if they are already a cigarette smoker. If they aren’t, they won’t be given further information encouraging them to use IQOS.
By contrast, Juul devices and pods are sold at a wide variety of convenience stores and vape shops, including 7-Eleven and Circle K, a wider system of distribution that makes it much tougher to keep the products out of the hands of teens. Vape shops and online outlets also sell gray-market pods that are compatible with Juul devices, an even tougher area for the company to police.
Reduced Risk?
While many people who vape say they’ve successfully used the products to quit smoking, Juul and many of its rivals have yet to clinically prove to the FDA and international regulators that their devices are a safer alternative to cigarettes. IQOS still doesn’t have approval to market itself as less risky than cigarettes in the U.S. either, but the FDA has at least reviewed what’s in it it and seen studies from Philip Morris that the company says show it has fewer toxicants than cigarettes.
Altria Chief Executive Howard Willard said Wednesday that some state legislative bodies have even advanced tax policies that might encourage smokers to switch to IQOS or other nicotine products -- if they are found by FDA as presenting lower risk.
As Juul stops all advertising, it may signal the end of the Wild West in cigarette alternatives, but that doesn’t mean there won’t be future competition for IQOS.
“We know that conversion will only happen if adult smokers find the products to be satisfying,” Willard said. “No single product is likely to meet the preferences of all adult smokers.”
--With assistance from Scott Deveau, Corinne Gretler, Hema Parmar and Jonathan Roeder.
To contact the reporter on this story: Tiffany Kary in New York at tkary@bloomberg.net
To contact the editors responsible for this story: Crayton Harrison at tharrison5@bloomberg.net, Anne Riley Moffat, Timothy Annett
©2019 Bloomberg L.P.