Pfizer Won the First Round, But Moderna Sees Final Victory Ahead
“I don’t intend to beat them at their game,” Moderna President Stephen Hoge said. “They’re trying to figure out how to play mine.”

(Bloomberg) --
Pfizer Inc. has been first across the finish line in nearly every leg of the Covid-19 vaccine race, but Moderna Inc. executives say their company’s long experience with messenger RNA technology give it a more enduring advantage.
“We’re not dabbling” in mRNA, the technology that is the backbone of both the leading U.S. vaccines, Moderna President Stephen Hoge said in an interview on Friday. “We’ve been doing this for a few years, we’re the creators of this space.”
Hoge said Pfizer’s experience with fast clinical trials and mass production allowed it to excel in the short run. The pharmaceutical giant, founded in 1849, has developed countless household-name therapies. Additionally, its partner, BioNTech SE, had been working on mRNA for years.
Moderna was started in 2010 and has just one product on the market, its vaccine. But Moderna’s superior technology and RNA know-how will allow it to pull ahead as the market matures, Hoge predicted.
“I don’t intend to beat them at their game,” he said. “They’re trying to figure out how to play mine.”

Moderna has reached an important moment in its brief history. Its stock soared when it emerged as a Covid-shot frontrunner, but it’s facing increasing skepticism from investors who say its next products are years away from the market -- and that in the meantime, a deep sales slide could be in the offing for Covid vaccines.
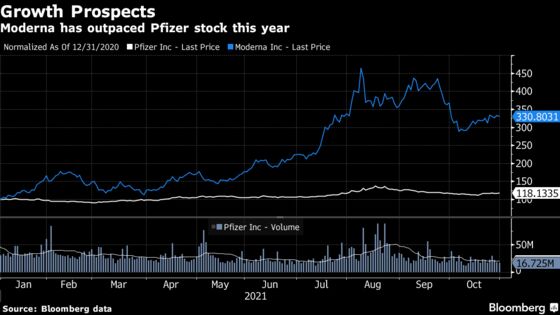
Indeed, that anxiety has put pressure on the shares of both Moderna and Pfizer. Moderna has dropped 29% since hitting a 52-week high on Aug. 9, while Pfizer has fallen by 13% since touching its high for the past year on Aug. 17.
Despite those fears, Moderna executives said they are sticking with the formula that made them one of the most richly valued startups in biotechnology history, and that their approach offers them plenty of flexibility to compete with bigger drugmakers.
Chief Executive Officer Stephane Bancel said the company, whose ticker symbol, MRNA, is a reflection of its commitment to its core technology, won’t rush to do deals to try to prop up sales.
“We’re not trying to do a deal to increase revenue. It’s not a revenue game. It’s a science and technology strategy that we have,” said Bancel. “We have a very dense pipeline and a lot of revenues coming from it.”
Production Gap
For the moment, however, Moderna is operating in its bigger rival’s shadow. Hoge acknowledged that Pfizer has stayed a month or so ahead in the initial Covid-19 vaccine and booster rollouts, something he said was a largely a function of Pfizer’s manpower and production heft. Moderna hadn’t manufactured anything at scale prior to the pandemic.
Pfizer and BioNTech plan to produce 3 billion vaccine doses this year; Moderna will make no more than a billion. The gap will get smaller next year, when Moderna aims to produce 3 billion doses, compared with Pfizer’s projected 4 billion.
“We’ve been told forever we couldn’t compete with Pfizer,” said Hoge. He said on a per-employee basis, Moderna is exceeding Pfizer’s output. “For the extra, whatever it is, 78,000 people, how come they are only a month ahead?”
“We have all worked tirelessly and collaboratively to combat the Covid-19 global health crisis, as we have known from the very beginning that it would take the efforts of more than one company to fight this deadly virus,” said Pfizer spokeswoman Amy Rose. “At Pfizer, we’re proud of all that we’ve done but we’re equally proud of what our peers have accomplished, too.”
Unlocking Combinations
Moderna also thinks it can outflank bigger players when it comes to other vaccines, because its reliance on mRNA will enable it to introduce combinations more quickly.
Bancel said Moderna is working on a combined flu and Covid shot that could be on the market by 2023-2024. The company is also targeting a three-way combination aimed at flu, Covid and respiratory syncytial virus (RSV), for which there is no current vaccine. Several companies, including Pfizer, are pursuing RSV vaccines that use different technology.
Since Pfizer’s RSV vaccine isn’t based on mRNA, it will make it much harder to combine with other messenger RNA shots, Bancel said.
“We’re operating in a very different space than traditional, large-scale, grind-it-out machines,” said Hoge. “We think we’re operating log orders more effectively and efficiently.”
Wall Street analysts expect Moderna’s revenue, now dependent almost entirely on its Covid shot, to drop after 2022 as the coronavirus becomes endemic. Bancel said he expects older people will continue to receive boosters, but it’s not clear yet how long the protection from the current vaccines and boosters will last for the young.
Still, the company isn’t looking to do the sorts of deals drug companies have long sought out when faced with a downturn in sales. Instead, it will focus on acquiring or licensing new technology that could supplement its mRNA platform, Bancel said.
In particular, Moderna won’t acquire traditional protein-based drugs or pills, Bancel said. But Moderna is interested in technologies involving nucleic acids like RNA interference, gene therapy and gene editing, he said.
Global Outcry
Moderna has also been under fire for not getting more doses of its vaccine to low-income countries. This week it agreed to a deal with the African Union that would send 110 million doses to the continent. And on Friday, it announced a pact to supply to 56.5 million additional doses to the Covax vaccine-distribution effort for low- and middle-income countries in the second quarter of next year.
Bancel said that one reason it hadn’t provided more doses to low-income countries was that until recently, the vast majority of its supply has been locked up by the U.S., European Union and other wealthy countries that had signed early contracts with the company.
Many of these countries are sitting on excess supply, Bancel said, adding that Moderna expects to forge more partnerships with low-income countries.
In the interview, Hoge acknowledged that it’s possible that Moderna’s vaccine has a slightly higher risk of the rare heart inflammation problems than the Pfizer vaccine. Hoge said the data isn’t conclusive whether there is a difference. If it does have slightly elevated risk, it could be related to Moderna’s higher dose, which likely also makes the shot more effective, he said.
©2021 Bloomberg L.P.