Sarepta Gains After Pfizer DMD Gene Therapy Data Fall Short
Sarepta Gains After Pfizer DMD Gene Therapy Data Fall Short
(Bloomberg) -- Pfizer Inc.’s experimental gene therapy for Duchenne muscular dystrophy helped boys with the deadly disease, but failed to match benefits previously shown by competitor Sarepta Therapeutics Inc.
All nine of the boys, ages 6 to 12, in the early-stage trial started to produce a key protein called dystrophin after receiving the one-time treatment. While patients with DMD don’t normally make any of the protein needed for muscles to work properly, three boys on a higher dose of the gene therapy produced about 52% of normal levels a year after treatment, Pfizer said in a statement.
One boy on the higher dose needed platelet transfusion and treatment with Alexion Inc.’s Soliris to boost the number of platelets in his blood after his immune system reacted to the gene therapy. While he fully recovered, the reaction marks the third serious adverse event tied to the treatment after two other patients were hospitalized shortly after infusion. In response to the previous safety concerns, Pfizer adjusted its study to increase monitoring and management, which the company says helped mitigate the most recent reaction.
The positive results for Pfizer’s therapy would be “a home run for DMD patients, in a vacuum,” wrote Baird analyst Brian Skorney. He said the update “just looks like a pale comparator to Sarepta’s SRP-9001” as the benefits appear lower than those seen with Sarepta’s therapy, and safety issues raise red flags.
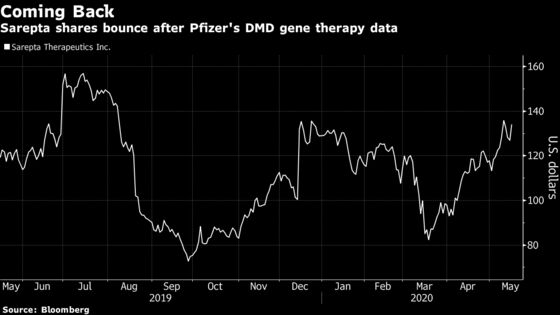
Sarepta shares jumped as much as 8.6% Friday, the biggest intraday gain for shares since late March, while Pfizer stock was little changed. Shares of the Cambridge, Massachusetts-based biotech are up 15% in the past year.
While analysts had been looking forward to an update on the competitive landscape for DMD gene therapies, comparison remains difficult. While Sarepta has relied on a standard analysis called Western Blot that measures the level of dystrophin expression, Pfizer has opted for different technology. Measured by Pfizer’s test, the average expression of patients on the higher dose was significant compared to a baseline measure, with five of the six boys showing an increase in mini-dystrophin concentration between two and 12 months.
The gene therapy’s benefit was also measured through a standard test of movement ability, including things like walking and climbing. While boys in the 7- to 9-year-old range typically start to plateau or lose motor abilities, six patients on Pfizer’s therapy for at least a year posted 3.5-point gains.
A low-single-digit improvement in that score would be “somewhat encouraging” for the field, Bernstein analysts said on Thursday, as it would suggest dystrophin production translates to a functional benefit. At a conference in June, Pfizer said two boys in its trial posted a 4.5-point gain one year after treatment.
DMD is a genetic disease characterized by progressive muscle degeneration and weakness. It’s the most common form of muscular dystrophy worldwide and affects about 250,000 people in the U.S., mostly boys. Pfizer’s data are being presented at a virtual meeting of the American Society of Gene & Cell Therapy.
In the Pfizer study, another exploratory analysis used MRI to show a reduction in fat in the thighs of boys treated at the higher dose after a year of follow-up. That suggests the gene therapy may have improved muscle fiber health and quality, Pfizer said, noting that DMD patients typically lose muscle and gain fatty tissue as the disease progresses. No reduction in fat was seen in patients receiving the lower dose.
The New York-based drugmaker plans to start treating patients in a late-stage study in the second half of the year after it gets clearance from the U.S. Food and Drug Administration. The company is neck-and-neck with Cambridge, Massachusetts-based Sarepta, which expects to start a multicountry study later this year after delays. SVB Leerink analyst Joseph Schwartz wrote earlier this month that “investors will likely reward the first company out of the Phase 3 gates.”
©2020 Bloomberg L.P.