Pfizer Roars to Record on Covid Vaccine, Treatment Successes
Pfizer Roars to Record on Covid Vaccine, Treatment Successes
(Bloomberg) -- Pfizer Inc. rallied to an intraday record after securing wider use of its Covid-19 vaccine, capping a week in which the drugmaker also scored another potential breakthrough with its pill to fight the disease.
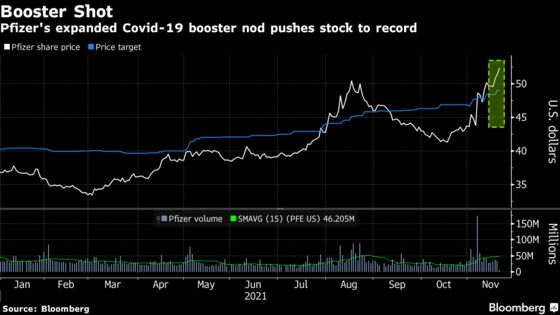
The shares rose 1.6% to $52.25 on Friday, topping the previous high of $51.86 set on Aug. 18.
The U.S. Food and Drug Administration expanded authorization Friday for booster shots from Pfizer and its German partner BioNTech for all adults, while a panel of advisers to the Centers for Disease Control and Prevention is meeting to make its recommendations. Pfizer’s stock is up 42% this year after securing the first U.S. authorization for a Covid shot in December 2020.
Rival Moderna Inc. also received an authorization for its booster shots before the market opened, with its shares rallying as much as 9.3%. Wall Street is underestimating the longer-term potential for Covid-19 vaccines, with revenue estimates dropping off aggressively over the next few years, said Evan Seigerman, an analyst at BMO.
“We see the move to children, potential variant vaccines and booster shots giving Pfizer a longer sales opportunity than modeled,” he wrote in a note initiating coverage with an outperform rating on the stock.
Pfizer and BioNTech’s shot is now the best-selling medicine of all time in a given year, and is expected to rake in $36 billion in sales this year and an additional $29 billion in 2022. Pfizer’s forecast for next year put some investor concerns about its Covid sales projections to rest, Evercore ISI’s Umer Raffat said after the company posted third-quarter earnings in early November.
Covid Pill
While the heady retail trader-driven enthusiasm for Moderna has cooled of late, Pfizer has had its own successes advancing shots into children as young as five and in promising data for a new Covid treatment pill, Paxlovid. The New York-based company asked U.S. regulators to give an emergency use authorization for the oral medicine on Nov. 16, and key readouts in non-hospitalized high-risk as well as symptomatic people are expected before the end of the year.
Further supporting vaccine stocks, U.S. President Joe Biden’s administration is offering drug manufacturers, including Pfizer and Moderna, funding to expand domestic production capacity of mRNA vaccines by one billion doses a year by the second half of 2022.
The stock’s ascent, unlike those of its biotech peers, has been less volatile even if more modest. More than 75% of its stock is held by investment advisers.
Wall Street is cautious as the stock’s rally has pushed it past the average analyst price target of $49, with 10 buy ratings, 14 holds and one sell recommendation.
©2021 Bloomberg L.P.