Pfizer, BioNTech Shares Ripe for a Bumpy Ride Ahead of Panel
Pfizer, BioNTech Shares Ripe for a Bumpy Ride Ahead of Key Panel
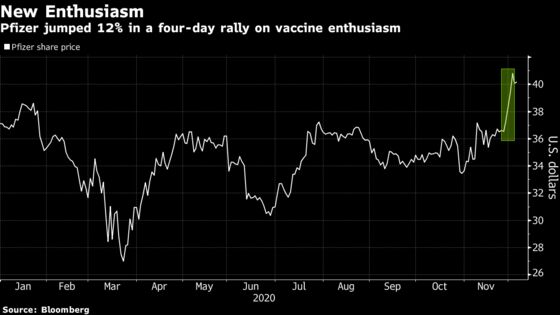
(Bloomberg) -- It’s been a wild ride for Pfizer Inc. shares as the S&P 500 heavyweight got caught up in the vaccine-stock euphoria. A key panel next week could mean even more volatility.
A Food and Drug Administration advisory committee meeting scheduled for Dec. 10 is believed to be a means for the agency to assure the public about Covid-19 vaccine safety, and a U.S. emergency use authorization could follow within days.
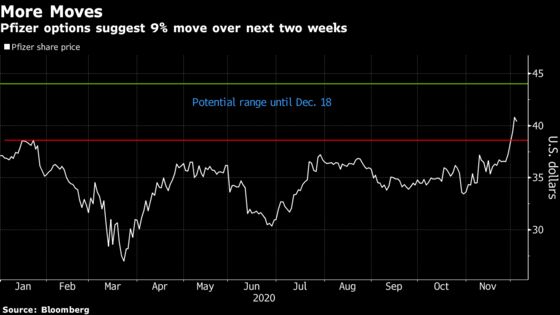
Pfizer shares have gained almost 20% since late October on optimism that its shot will be approved. Options prices imply a more than 9% move over the next two weeks. On Friday, Pfizer was little changed, trading near the 17-month high it reached earlier in the week.
BioNTech SE, Pfizer’s partner in developing the shots, could move nearly twice that much.
Read more: Pfizer Covid Shot Distribution Plans Due From States Friday
While investors are accustomed to large price swings in the biotech sectors, it’s unusual to see a move of such magnitude in an established pharmaceutical giant such as Pfizer, which has more than $220 billion of market value.
Bearish bets against Pfizer have been rising, with nearly 62 million shares sold short. That’s the most of any S&P 500 stock by that measure, according to S3 Partners data. Among biopharma companies, that $2.5 billion short position is the second-largest behind only its closest competitor in the race to develop a Covid-19 shot, Moderna Inc.
Short Bets
Pfizer shorts have fared much better than Moderna bears, they were up by roughly $55 million for far this year as of Thursday evening. “Any shorts that might have thought about heading for the exits because of November and early December mark-to-market losses just turned on their heels and went back into the theater to see how the picture ends,” Ihor Dusaniwsky, managing director of predictive analytics at S3 said in an interview after Pfizer sank 1.7% in the final hour of yesterday’s session.
An analysis of options in Pfizer set to expire on Dec. 18 shows shares are expected to move by about 9.4%, and implied volatility is elevated at around 58 versus a three-month average of 27. BioNTech may swing as much as 18%, the data suggest.
For Moderna, which has a panel set for the following week, options show shares are expected to move by about 22% between now and Dec. 18 with implied volatility around 135, compared with a three-month average of 76.
While authorization for Pfizer and German partner BioNTech’s vaccine is widely expected, the question remains how much a shot will boost the New York City-based company’s sales over the long-term.
Pfizer may get a boost in sales in the next year or two “the sustainability of that revenue stream is in doubt” with competitors waiting in the wings to develop shots that may be easier to transport or administer, said Barclays analyst Carter Gould.
“There’s definitely a retail component to this” and if there were an unwinding after an authorization that wouldn’t be surprising, he said. He has a Wall Street-low price target of $33.18 on the stock which implies a roughly 17% downside to today’s trading.
©2020 Bloomberg L.P.