Peanut Allergy Drug Faces Next Test at Key FDA Panel Meeting
Peanut Allergy Drug Faces Next Test at Key FDA Panel Meeting
(Bloomberg) -- Aimmune Therapeutics Inc.’s winding road to get its first allergy treatment approved by U.S. regulators faces its next turn when a panel of advisers to the Food and Drug Administration meets later this week.
The advisory committee’s discussion and recommendation on Sept. 13 may guide investors seeking details about the potential commercial success of a product designed to protect patients with peanut allergies.
Bullish analysts expect the panel to back the drug, AR101, and its ground-breaking potential. They are looking for such an endorsement to lift shares, which have shed more than 30% of their value since a November high. At recent levels, some Wall Street price targets project Aimmune could double or triple in price over the next 12 months.
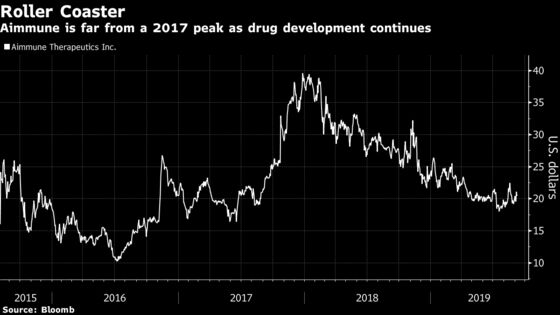
Among skeptics is Stifel’s Derek Archila, who is hesitant to advise clients to buy shares before the “binary catalyst” of the advisory committee. Archila in a research note wrote that he expects the panel to focus on the safety of AR101, which is derived from peanut flour. A negative vote could cut the value of Aimmune’s shares in half, while a positive review from the advisers may prompt a 30% climb.
“The FDA is really going to be charged with whether it can get comfortable with the longer-term safety,” he said in a telephone interview. “The crux of the AdComm will be if there is a need for longer-term safety data to really understand if you’re putting these kids at undue risk by giving them the allergen they’re allergic to.”
Jayson Dallas, chief executive officer of Aimmune, said in an interview at Bloomberg’s New York headquarters: “There almost surely will be discussion around the macro-level risk/benefit, but then maybe digging into some patient populations.”
A successful panel meeting followed by U.S. clearance over the coming months would make Aimmune’s AR101 the first desensitization therapy approved for an allergy that affects more than 1.6 million kids and teens and can produce potentially life-threatening reactions.
When thinking about the company’s sales potential, Dallas mentioned GW Pharmaceuticals Plc’s cannabinoid-based drug Epidiolex as a comparison.
“They were the only player in town, with a high unmet need and clear pent-up demand,” but sales didn’t really pick up until insurance coverage started to take hold, Dallas said. Epidiolex brought in $68.4 million of U.S. sales for the three months ended June 30, compared with $33.5 million for the prior quarter.
While U.S. regulators review Aimmune’s treatment, a nonprofit research group has already weighed in. The Institute for Clinical and Economic Review (ICER) in a July report said there isn’t enough evidence to show that the “long-term benefits of desensitization outweigh short-term risks.”
The company, along with analysts like Piper Jaffray’s Christopher Raymond, criticized the report at the time. Raymond called it “yet another example of ICER’s inherent bias against any novel therapy.” Aimmune said “ICER fails to incorporate available data on both long-term outcomes and quality of life.”
If AR101 does win approval, analysts project annual sales could exceed $1.2 billion by 2024, data compiled by Bloomberg show. RBC Capital Markets’ Kennen MacKay wrote in an August note that the majority of U.S. allergists are waiting for an FDA-approved therapy to offer patients, which could push sales toward $1.9 billion worldwide.
Another aspect that could affect the uptake of AR101 is whether the FDA will require a safety program to help ensure the benefits outweigh its risks. The company’s CEO said such a plan would “not be a big deal” because Aimmune is planning to assess patients over longer periods of time and look for larger groups of patients.
An approval would likely come with a “black box” warning, the FDA’s most serious caution, according to both Aimmune’s Dallas and Stifel’s Archila, though this would be in-line with other allergy medicines.
If Aimmune can find market success fighting peanut allergy, that might bode well for its experimental approaches to egg and tree-nut allergies. The Brisbane, California-based company enrolled the first patient in a mid-stage egg-allergy study at the end of August.
“Validation of the platform and its concept will speed up our egg program and create more excitement for the egg program and our tree nut program,” Dallas said.
Options in Aimmune set to expire on Sept. 20 are expecting a 36% move in shares over the next two weeks. Traders appear to be hedging more than making bullish bets, with put options outweighing calls. Investors are also preparing for an outsized move with implied volatility sitting at about 259%, well above a three-month average of 41%.
This month’s FDA allergy panel may have implications for DBV Technologies SA, which is developing a patch for peanut-allergic children ages four to 11. DBV last month submitted its application for Viaskin Peanut after the FDA had previously requested additional data.
While the FDA typically heeds the advice of its panels, the agency isn’t obligated to follow it. Karyopharm Therapeutics Inc.’s blood cancer drug Xpovio is a recent reminder. Regulators cleared the drug for market four months after a panel voted that a decision should be delayed for more data.
--With assistance from Gregory Calderone.
To contact the reporter on this story: Bailey Lipschultz in New York at blipschultz@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Jeremy R. Cooke
©2019 Bloomberg L.P.