Patients Want Biogen’s Alzheimer’s Drug and Someone Has to Pay
Patients Want Biogen’s Alzheimer’s Drug and Someone Has to Pay
(Bloomberg) -- More than a hundred Alzheimer’s disease patients are lined up to take Biogen Inc.’s new drug at Butler Hospital in Providence, Rhode Island, the first center to administer it outside a clinical trial.
Controversy over how Biogen’s Aduhelm was cleared and whether it actually slows Alzheimer’s brain-wasting impact hasn’t deterred them. Rather, the biggest obstacle for many patients will be whether their insurance will cover the drug’s $56,000 annual cost.
“The main thing that is delaying the rollout is the uncertainty about insurance coverage,” said Stephen Salloway, director of neurology at Butler, where four patients have begun monthly infusions.
In a rare conflict with a panel of its external advisers, the Food and Drug Administration approved Aduhelm June 7, citing evidence that it removes a harmful brain protein linked to Alzheimer’s disease. While one of two large trials suggested the treatment slightly slowed the rate at which patients deteriorate, another conducted at the same time showed no clinical benefit. Now the drug’s success hinges on whether public and private insurers will pay for it, based on the scant, conflicting data.
At a public meeting in July, Salloway urged the U.S. Centers for Medicare and Medicaid Services to cover the drug. The agency began a process that may take until April to assess whether and how Medicare, the federal health program for the elderly, will reimburse for Aduhelm. Large private insurers like UnitedHealth Group Inc. have said they’re seeking guidance from the agency to shape their own coverage decisions.
Until CMS makes a national decision about coverage, local Medicare contractors who are hired to process claims will decide whether to pay for the drug on a case-by-case basis. “CMS is in a pickle,” said Peter Bach, a drug-pricing expert at Memorial Sloan Kettering Cancer Center in New York.
Bach said the agency has a range of options for how to handle the drug, including restricting coverage to people in a randomized trial to assess how it works. “If anything, the treatment effects are very small, and the toxicities are very considerable,” Bach said. “You’re going to need very precise estimates of both,” and careful randomized trials are the gold standard, he said.
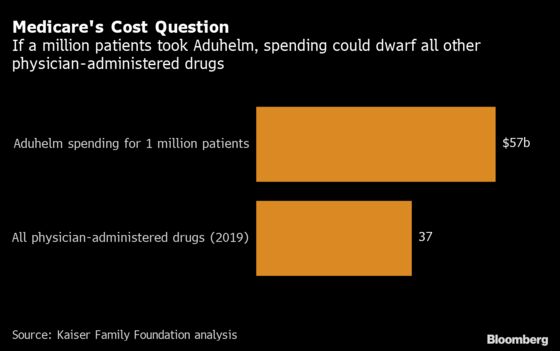
Biogen has said as many as 2 million Americans might qualify for Aduhelm, though it expects fewer to actually get it. Still, if just half that many patients get it, the $57 billion annual bill including drug costs and other expenses would exceed what Medicare pays for all other medications administered by physicians, according to a Kaiser Family Foundation analysis.
The projected costs have alarmed lawmakers and economists. Yet cost isn’t supposed to be a factor in Medicare’s coverage decision; officials are expected to determine whether the drug is “reasonable and necessary.”
“They are thinking about the clinical evidence involved,” said said Rachel Sachs, a law professor at Washington University in St. Louis. “They are not thinking about the price of the drug.”
Biogen Chief Executive Officer Michel Vounatsos called the launch “a bit slower than what we assumed” in a call with Wall Street analysts July 22. The company expects “modest” Aduhelm revenue this year, increasing in the years to come.
Some private Medicare Advantage plans, which contract with the federal program to cover seniors, have already approved prior authorization requests for Aduhelm.

A Biogen spokesman declined to comment on which plans have approved coverage of the drug or whether Medicare has begun reimbursing for it. A CMS spokesperson wouldn’t comment on whether claims have been approved.
The FDA granted Aduhelm accelerated approval in June based on its ability to clear the harmful protein, called amyloid, from the brain. It ordered Biogen to do another study to confirm the treatment benefits patients by slowing the rate at which they lose cognitive function. But that could take as long as nine years.
Three members of FDA’s scientific advisory panel resigned after the approval, and U.S. health authorities and congressional committees are investigating the process. The Institute for Clinical and Economic Review, a nonprofit that evaluates medications, has said there’s insufficient evidence for Aduhelm’s benefit.
The American Academy of Neurology, a 36,000-member group of doctors and researchers, warned about the costs of administering Aduhelm widely while its ability to help patients remains uncertain. The impact on Medicare spending “cannot be overstated as aducanumab could cost more than a trillion dollars before its clinical benefit is adequately demonstrated,” the group said in a comment to ICER.
Some hospital systems, including Cleveland Clinic and Mount Sinai Health System, have said they won’t administer Aduhelm, citing uncertainty about the evidence. But even providers ready to get it to patients are in limbo, concerned they might not get paid for the costly medicine and associated fees for administering it.

“We are ready to infuse the drug. My nurses have been trained, the protocols have been created,” said Christine Mann, chief operating officer at Dent Neurologic Institute in western New York. “The problem is we don’t have insurance providers or Medicare approving the drug.”
It’s not for lack of interest, Mann said. Appointments with the center’s memory specialist for evaluations are booked into October. But unless patients are prepared to pay out-of-pocket, the center is waiting to confirm whether an insurer will cover Aduhelm treatment.
Until the coverage rules are clarified, any patient taking Aduhelm could be at risk of being billed for the full price. “The difficult conversation we have with the patients right now is, we can’t tell somebody what their cost share would be,” Mann said.
And Biogen wants the payment gatekeepers to know that the clock is ticking. About a thousand Americans each day progress from early-stage Alzheimer’s to more moderate disease, CEO Vounatsos said on the company’s earnings call, which could disqualify them from treatment with Aduhelm.
©2021 Bloomberg L.P.